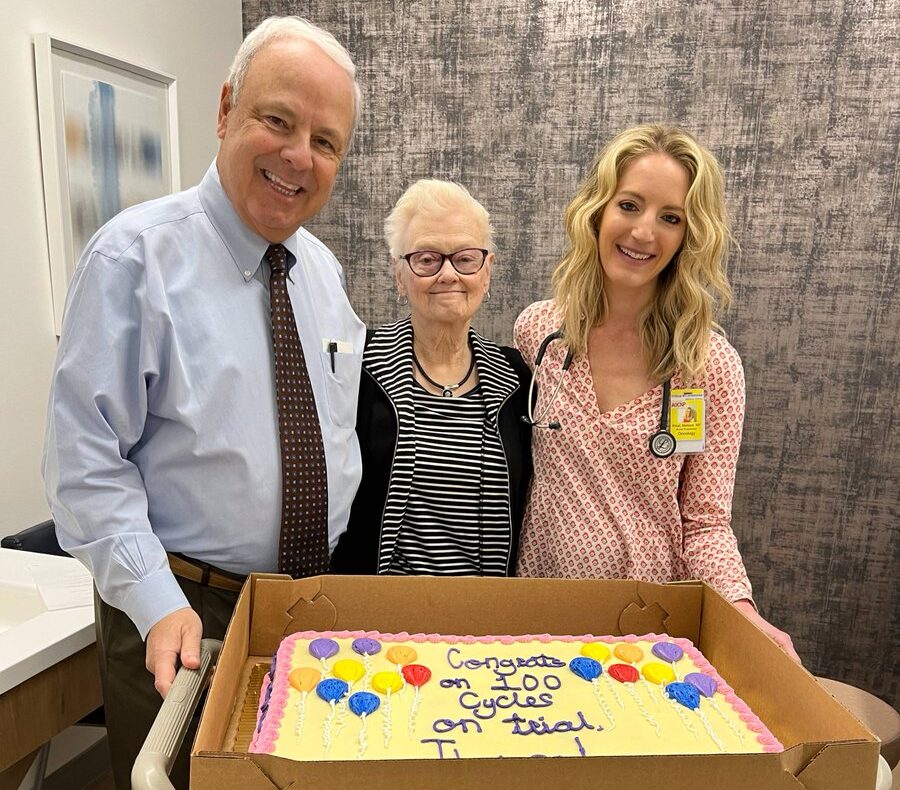Erika Hamilton and shared a post by
Following further testing, Theresa’s primary doctor explained that she was a candidate for a clinical trial at Sarah Cannon Research Institute (SCRI) in Nashville, Tenn., where she met Erika Hamilton, MD, Director, Breast Cancer Research; Executive Chair, Breast Cancer Research Committee, SCRI, and began her clinical trial treatment journey under Dr. Hamilton’s supervision using a hormone blocker in combination with a PI3 kinase inhibitor in the first-line setting.
Quoting Vivek Subbiah on X on the topic:
“100 months on a phase 1 trial – Precision Oncology in action. Delivering the right medicine to the right patient at the right time.
Several years ago, Theresa noticed a knot in her right breast, but at the time, she was caring for her mother and delayed visiting her doctor. Following her mother’s passing, Theresa noticed a new lump growing on the crown of her head and scheduled a biopsy with a dermatologist, which revealed a malignant growth.
This ultimately led to her being diagnosed with stage 4 metastatic breast cancer Following further testing, Theresa’s primary doctor explained that she was a candidate for a clinical trial in SCRI, where she met Erika Hamilton and began her clinical trial treatment journey using a hormone blocker in combination with a PI3 kinase inhibitor.
Theresa continues the trial with no visible disease on scans & has maintained a thriving & full life in her retirement. In fact, Theresa recently celebrated 100 months on her phase 1 clinical trial, Skip Burris and the care team at SCRI in Nashville surprised her with a cake to honor this incredible moment.”
Source: Erika Hamilton/LinkedIn, Vivek Subbiah/X and Sarah Canon Research Institute/LinkedIn
Erika Hamilton is a medical oncologist and the Director of Breast Cancer and Gynecologic Cancer Research at Sarah Cannon Research Institute (USA). In her role, Dr. Hamilton oversees the research program and clinical trial menu for gynecologic and breast cancer from a medical oncology perspective. Dr. Hamilton is a past chair of ASCO’s Scientific Breast Committee a ’21-’22, participant of the ASCO Leadership Development Program, Associate Editor for Clinical Breast Cancer, co-chair for Great Debates and Updates in Women’s Oncology Conference and a board member of the Susan G. Komen Foundation of Central Tennessee. She is active in the community, volunteering with Gilda’s Club Middle Tennessee, the American Cancer Society’s Hope Lodge, Ovarcome ovarian cancer support organization, Brentwood Baptist Church and the Susan G. Komen Race for the Cure.
Vivek Subbiah is the Chief of Early-Phase Drug Development at the Sarah Cannon Research Institute (USA). He is the former Executive Director of Oncology Research at the MD Anderson Cancer Network and a former Associate Professor in the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center. Dr. Vivek Subbiah has served as the principal investigator in over 100 phase I/II trials and co-investigator in over 200 clinical trials and is known for his leadership in several first-in-human and practice-changing studies that directly led to approvals from the FDA, European Medicines Agency, and other agencies across the world. He is an expert in tumor agnostic precision oncology and leads the BRAF and RET tissue agnostic studies to FDA approval.