Imlunestrant has been approved for the treatment of breast cancer. It is a next-generation, brain-penetrant, oral selective estrogen receptor (ER) degrader that provides continuous ER inhibition, including in cancers with mutations in the ESR1 gene encoding ERα.
On December 11, 2024 the results of the EMBER3 Study were published in The New England Journal of Medicine. The results were presented at SABCS24.
Healthcare professionals shared their insights on social media:
“EMBER3 study results at SABCS24.
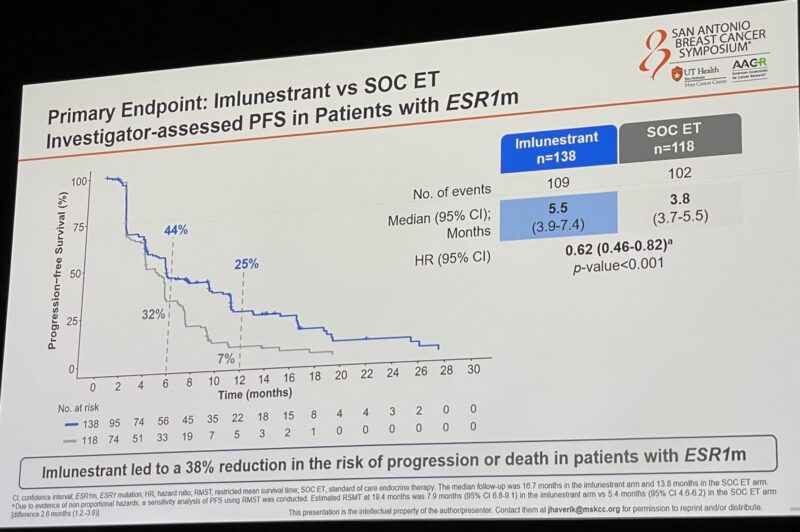
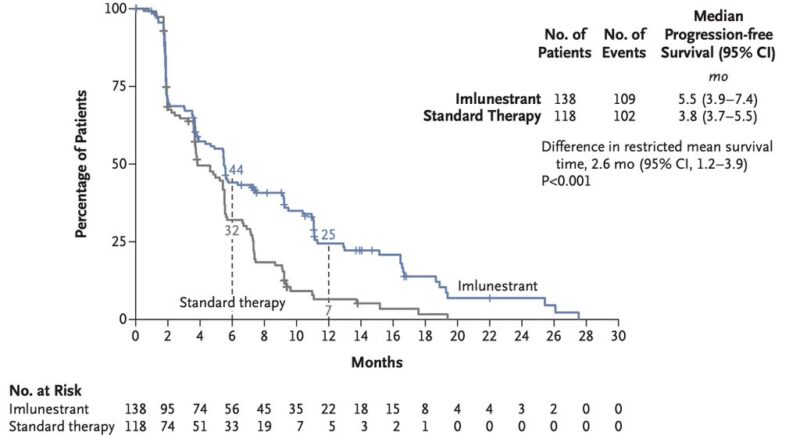
Imlunestrant significantly improved PFS vs SOC ET in patients with ESR1m.
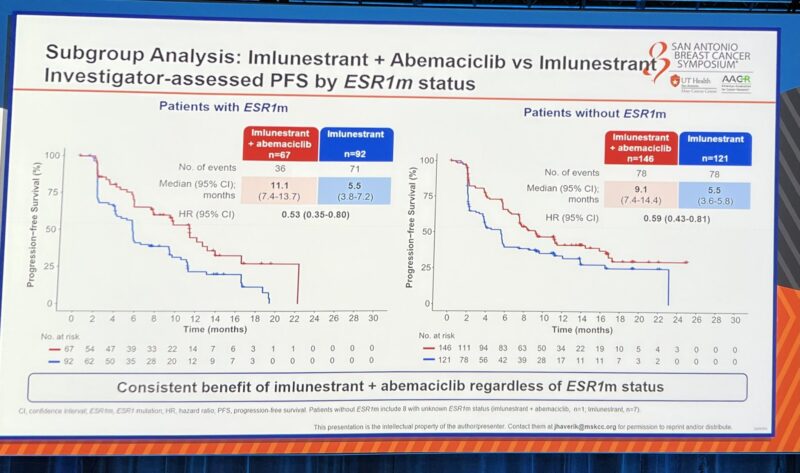
Imlunestrant plus abemaciclib improved PFS as compared to Imlunestrant alone regardless ESR1 status.”

“Love Harold Burstein caveat to this slide…but does suggest that maybe the sweet spot is:
Both – 1 CDK switch (and continued blockade) and 2 novel next-gen endocrine agent.”
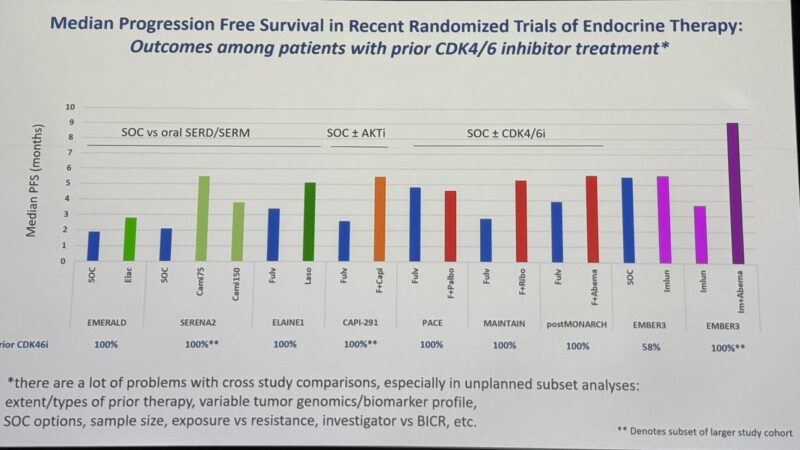
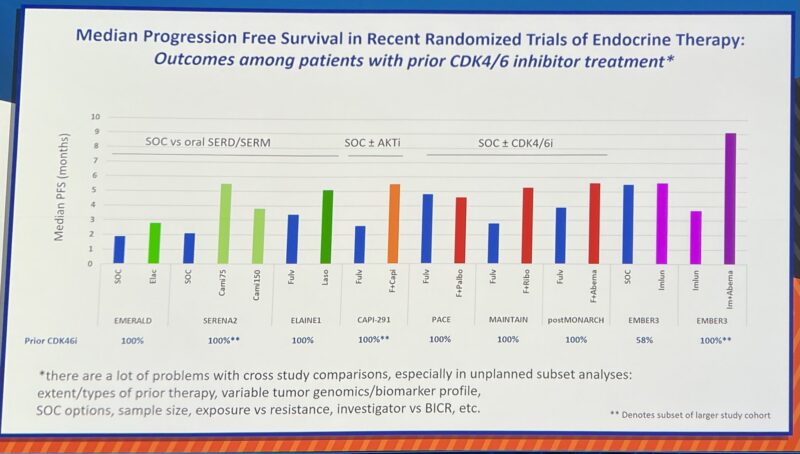
“Hal Burstein from Dana-Farber’s Breast Oncology Center puts the EMBER3 data in the context of other treatment options in ET-pretreated HR+/HER2- MBC.”
” ‘It is impressive that the combination (of imlunestrant and abemaciclib) seems to tower above the rest’ – Harold Burstein, sharing his self-proclaimed dreaded cross-trial comparison (students, biostatisticians: avert your eyes).”
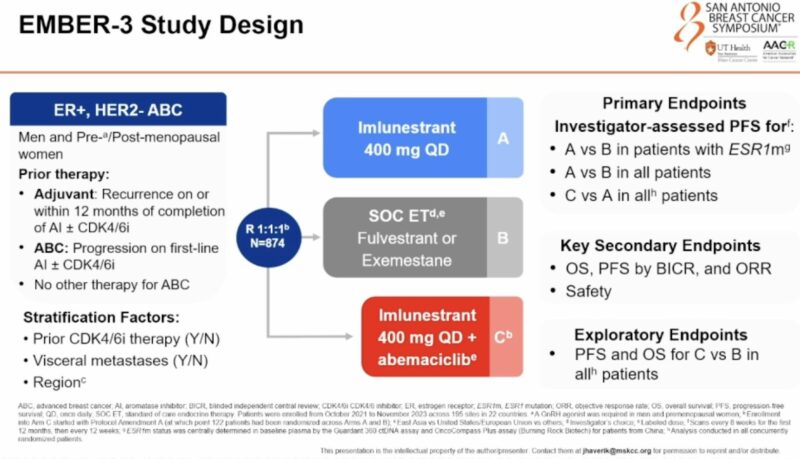
“Practice Changing EMBER3 trial.
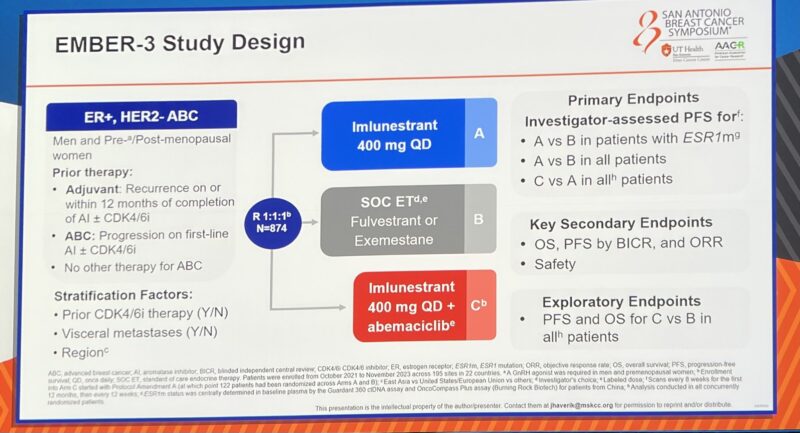
Oral SERD Imlunestrant plus Abemaciclib in MBC HR+ HER-2 – in 2L vs SOC ET.
Superior efficacy in patients with ESRm as /single agent or without ESR1m in Combination with abemaciclib!
Patients were exposed to previous CDK4/6i ~60% palbociclib.”
“Exciting Results from EMBER3!
Imlunestrant boosts PFS:
- As monotherapy in ESR1m patients
- In combo with abemaciclib, irrespective on ESR1 status
Patients exposed to ET+CDK4/6i (palbo 60%)
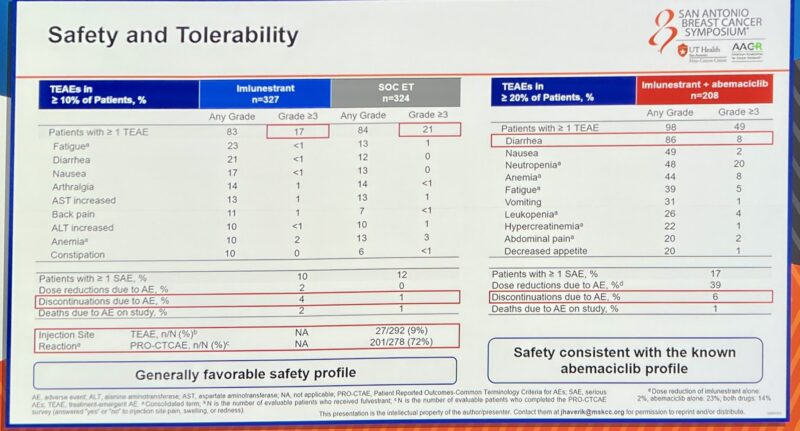
Well tolerated with low discontinuation rates.”
“My first takeaway from SABCS24. The EMBER3 of new oral SERD imlunestrant vs fulvestrant or exemestane vs imlune and abemaciclib in HR+/HER2neg MBC. This study showed in II-line scenario that imlunestrant is superior to ET alone in ESR1mutant patients. AKA welcome to elacestrant 2″
“Practice-changing data from EMBER3 presented by Komal Jhaveri and concomitantly published on NEJM. Imlunestrant and abemaciclib likely to become soon a new 2L treatment option for HR+/HER2- MBC. Major question: should this option be for all-comers or only patients with ESR1mut tumors?”

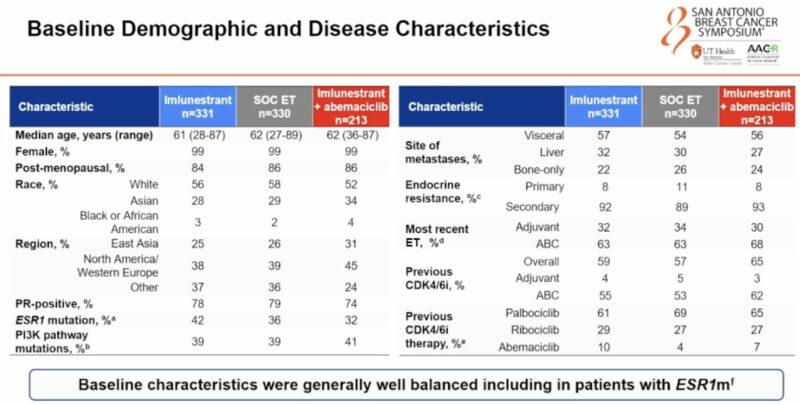
“EMBER3 shows benefit of imlunestrant in patients with HR+ MBC and mESR1 and for the combination of imlunestrant and Abemaciclib for all patients, regardless of ESR1 status.
Interesting subgroup breakdown shows the combo benefit persists independent of ESR1 status, prior CDK4/6 inhibition, and shows signal for CNS effect (very small numbers).
And the safety profile of imlunestrant looks good—minimal GI toxicity, no class toxicity concerns. The combo AEs are what we expect with abema.”
“EMBER3 takeaways:
- Imlunestrant high PFS in patients with ESR1 mut MBC (5.5 vs. 3.8 mo)
- Combination with abemaciclib further high PFS regardless of ESR1 and PIK3CA ctDNA status (9.4 vs. 5.5 mo)
- Previous CDK4/6i were mainly Palbo and Ribo in the MBC setting.”
Read the post “Imlunestrant and Abemaciclib: A Study in Advanced Breast Cancer” on oncodaily.com.


