Sergio Cifuentes Canaval, Cancer Research Project Manager at CENEIT México, shared a post on LinkedIn:
“Breast cancer remains one of the next frontiers for immuno-oncology.
From checkpoint inhibitors in TNBC to emerging strategies like CAR-T and TIL therapy, the field is evolving fast.
We invite you to read this article exploring current advances and future directions in breast cancer IO.
Immuno-oncology in Breast Cancer: Progress, Gaps, and Research Opportunities
Immuno-oncology (IO) is one of the most dynamic and expanding fields in cancer treatment. By harnessing the power of the host’s adaptive and innate immune systems, IO therapies aim to restore or enhance the immune response against tumor cells. Since the first approval of checkpoint inhibitors in 2011, this class has become the second most approved category of oncology therapeutics by the FDA.
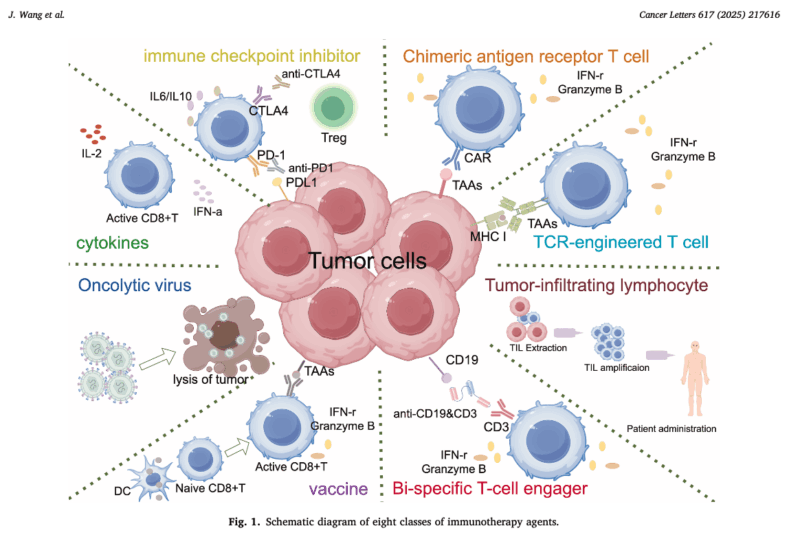
Currently approved IO modalities include immune checkpoint inhibitors (ICIs), CAR-T cell therapies, bi-specific T-cell engagers (BiTEs), T-cell receptor (TCR)-engineered T cells, tumor-infiltrating lymphocyte (TIL) therapy, cytokine therapy, cancer vaccines, and oncolytic virus therapies. While substantial clinical benefit has been achieved in diseases such as melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), and urothelial carcinoma, breast cancer remains comparatively less explored—offering a rich ground for innovation and research.
The Case of Triple-Negative Breast Cancer (TNBC)
TNBC is the most immunogenic subtype of breast cancer and has become the central focus of IO development in this field. In 2019, the IMpassion130 trial led to the first immunotherapy approval in breast cancer: atezolizumab plus nab-paclitaxel for PD-L1–positive unresectable locally advanced or metastatic TNBC. This was followed by KEYNOTE-355, where pembrolizumab plus chemotherapy showed improved PFS in PD-L1 (CPS ≥10) positive mTNBC patients, and KEYNOTE-522, which demonstrated significantly better pCR and EFS when pembrolizumab was added to neoadjuvant chemotherapy in high-risk stage II–III TNBC. These findings have opened the door for immunotherapy integration into both metastatic and early disease treatment, but the benefit remains limited to specific patient subsets.
What About Hormone Receptor–Positive Disease?
Hormone receptor–positive (HR+) breast cancer, despite being the most common subtype, is still considered immunologically “cold.” Tumor immune infiltration is often low, PD-L1 expression is infrequent, and clinical responses to ICIs in unselected populations have been disappointing. However, ongoing trials are exploring ways to overcome this barrier through rational combinations with endocrine therapy, CDK4/6 inhibitors, and epigenetic modulators. In addition, emerging biomarkers—such as immune gene signatures, TMB, and stromal features—may improve patient selection and trial enrichment.
Next-Generation IO Strategies in Breast Cancer
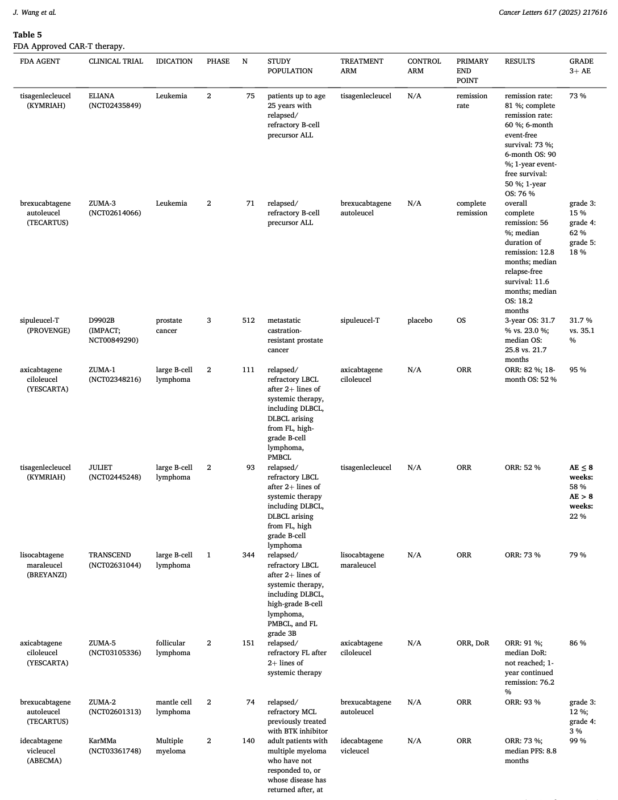
Though still in early stages, cell therapies like CAR-T and TCR-T are making inroads into solid tumors. Recent FDA approvals in hematologic malignancies—including CAR-T therapies for B-cell leukemias, lymphomas, and multiple myeloma—have created momentum for their application in epithelial tumors.
- TIL therapy is under investigation in metastatic TNBC.
- Cancer vaccines targeting HER2, MUC1, and mammaglobin are in clinical development.
- Oncolytic viruses, such as T-VEC, are being tested in combination with ICIs in advanced disease.
- TCR-T therapies, like afamitresgene autoleucel, recently approved for synovial sarcoma, provide a proof-of-concept that may be expanded into breast cancer with appropriate targets.
Opportunities for Clinical Research in Breast Cancer IO
Given the current landscape, several avenues for impactful research emerge:
- Incorporating IO into perioperative trials to evaluate long-term immunologic memory and recurrence prevention.
- Developing predictive biomarkers beyond PD-L1 and TMB to select patients more accurately.
- Exploring combinations with ADCs, radiotherapy, or DNA-damage response inhibitors to modulate the tumor immune microenvironment.
- Studying resistance mechanisms and immune evasion pathways specific to breast cancer.
- Addressing disparities in access to IO clinical trials, especially in low- and middle-income countries (LMICs).
LATAM and the Global IO Agenda
For researchers and clinicians in Latin America, participating in the immuno-oncology revolution represents both a challenge and an opportunity. Building research networks, contributing to global trials, and advocating for early access to innovative therapies are key steps toward improving equity in cancer care.
The next breakthroughs in breast cancer IO will come not only from major academic centers but from diverse, collaborative, and inclusive efforts worldwide.
Wang J. Clinical development of immuno-oncology therapeutics. Cancer Lett. 2025 May 1;617:217616. doi: 10.1016/j.canlet.2025.217616.”
Read more posts featuring Sergio Cifuentes.


