Aura Biosciences announced early results from a Phase 1 trial of its drug bel-sar (AU-011) for non-muscle invasive bladder cancer (NMIBC).
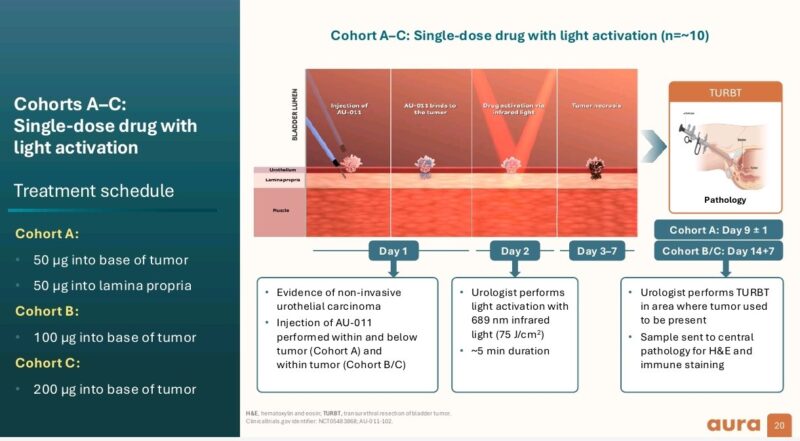
The trial, involving 13 patients, assessed the safety and feasibility of bel-sar administered alone and with light activation. Key findings include:
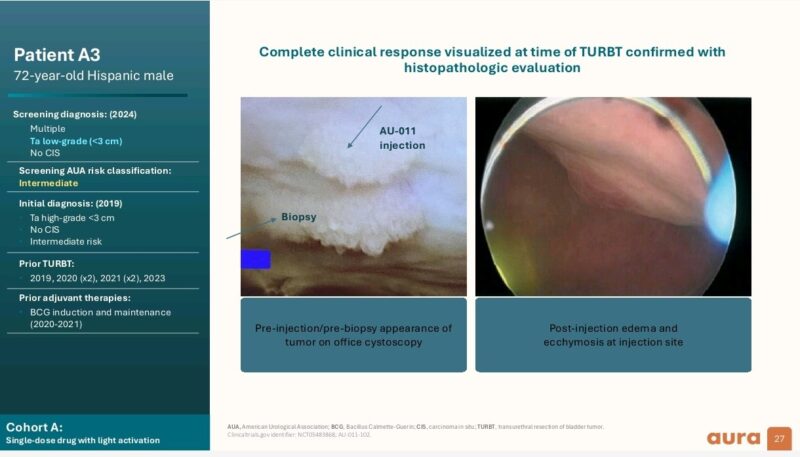
- 4 out of 5 patients with low-grade disease showed no tumor cells remaining.
- 2 out of 3 patients with high-grade disease exhibited visual tumor reduction.
- Only mild adverse events were reported, indicating a favorable safety profile
The trial also noted significant immune activation, including infiltration of CD8+ and CD4+ T cells in both treated and untreated tumors, suggesting a potential “bladder urothelial field effect” that could enhance treatment efficacy beyond the targeted tumors.
Aura Biosciences

Aura Biosciences is a Boston-based clinical-stage biotechnology firm focused on precision therapies for solid tumors, particularly through its lead candidate, bel-sar (AU-011).
Currently in late-stage development for primary choroidal melanoma, bel-sar has shown promising results with an 80% tumor control rate and 90% preservation of visual acuity in Phase 2 trials.
“We are highly encouraged by this positive early data, which shows that bel-sar has the potential to be a transformative cancer treatment. A potentially differentiating aspect of this novel treatment is the rapid tumor response accompanied by an immune oncology (IO) effect such as a marked CD8+ T-cell infiltration observed in just a matter of days with a single low dose. We believe this could have the potential to translate into a durable response. In parallel with expanding the ongoing Phase 1 trial, we are preparing for a Phase 2 trial to further evaluate bel-sar’s clinical activity and durability of response.” – Sabine Brookman-May, Senior Vice President, Therapeutic Area Head Urologic Oncology of Aura Biosciences
The company is also exploring bel-sar for bladder cancer and other ocular oncology indications. Aura aims to innovate and improve patient outcomes in oncology, with plans for a global Phase 3 trial underway.
“Bel-sar has the potential to change the treatment paradigm for NMIBC. Based on this early data, bel-sar’s positive clinical activity and evidence of a bladder urothelial field effect with a single dose, may position bel-sar to be the first immune ablative treatment option for early-stage bladder cancer patients delivered with an in-office procedure.” – said Neal Shore, Medical Director, Carolina Urologic Research Center, AUC Urology Specialists.
“This afternoon, Aura announced positive early data from an ongoing Phase 1 clinical trial of bel-sar (AU-011) in patients with non-muscle-invasive bladder cancer. Read the full release here.”

“BelSar AU011 phase 1 data presented today:
Clinical complete response in 4 out of 5 LG NMIBC patients
+ urothelial field effect in non-target lesions
Looking forward to further evaluating response and durability of response.
Data presentation was followed by a discussion of data with Joe Jacob, Max Kates, Gary Steinberg and Dr. Neal Shore – great chance to exchange with experts and bring data into context.”
Stay tuned by visiting oncodaily.com