Amol Akhade, Consultant Medical Oncologist Hemato-Oncologist at Suyog Cancer Clinics, shared on LinkedIn:
“CDK4/6 Rechallenge Trials: What Works and What Doesn’t?
published in JCO – The PALMIRA Trial brings clarity!
PALMIRA Trial (2025, JCO)
Rechallenge with palbociclib + new ET vs. ET alone
Result: No significant PFS benefit
PFS: 4.9 vs. 3.6 months
HR: 0.84 | p = 0.149
Conclusion: Same-drug rechallenge fails
Switch Strategy Trials Deliver
MAINTAIN Trial
Switched to ribociclib
PFS: 5.3 vs. 2.8 months
HR: 0.57 | p = 0.006
postMONARCH Trial
Switched to abemaciclib
PFS: 6.0 vs. 5.3 months
HR: 0.73 | p = 0.02
Switching CDK4/6 inhibitors = Real benefit
ESR1 Mutations Matter
Post-CDK4/6i ESR1 mutation rate: 30–47%
ESR1 mutations drive AI resistance
Oral SERDs (e.g., elacestrant) are effective in this population
Key Takeaways
- Palbociclib rechallenge ≠ clinical benefit
- Switching to ribo or abema = better PFS
- Precision matters: molecular profiling is the future
- Practice-changing evidence is here
Let’s move from one-size-fits-all to personalized CDK4/6i strategy in HR+ MBC!”
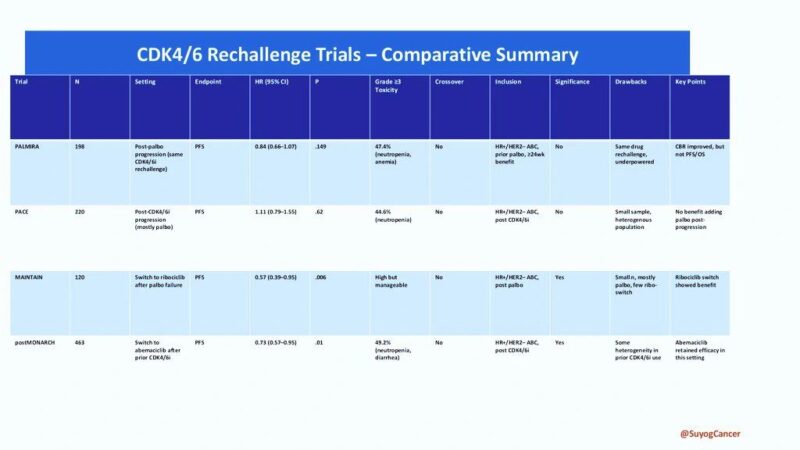
Amol Akhade reviewed CDK4/6 Rechallenge Trials.
He concluded that rechallenging with palbociclib did not yield significant benefit, while switching to ribociclib or abemaciclib showed improved outcomes. He emphasized the role of ESR1 mutations and the importance of molecular profiling in HR+ metastatic breast cancer.
More posts featuring Amol Akhade on OncoDaily.


