Alexander González, at 3cert Limited, shared on LinkedIn:
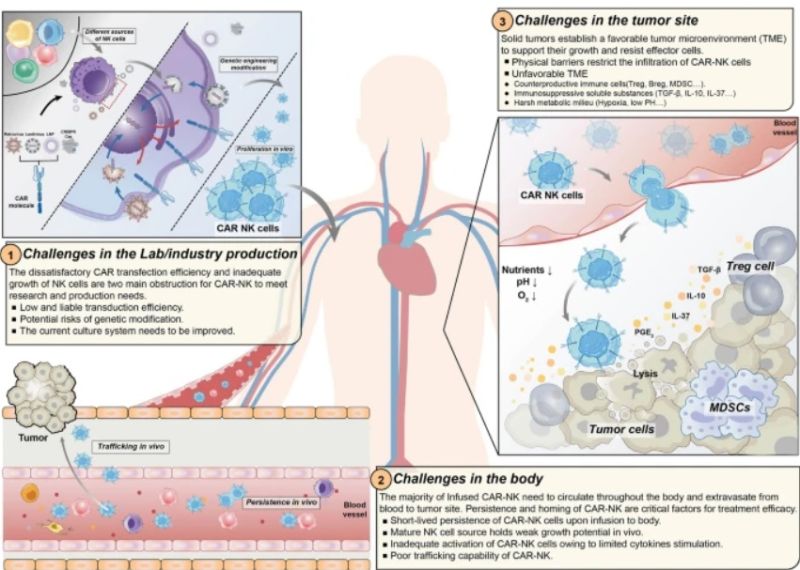
“The challenges of CARNK existing in the process from lab production to tumor infiltration.
In the image:
The unsatisfactory CAR transduction efficiency and limited proliferation ability add barriers to CAR-NK production. Multiple approaches including virus-mediated and non-viral-mediated transduction have been utilized to boost CAR expression and stability.
The ex-vivo expansion are mainly stimulated by cytokines or feeder cell system with limited potential. Upon infusion into the body, the trafficking and infiltration abilities are impeded by the disruptive chemokines/chemokine receptors axis in the dysregulated tumor vasculature.
In tumor bed, suppressive cells (Treg cells, Breg cells and MDSCs) and soluble inhibitory cytokines (TGF-β, IL-10 and IL-6) can disrupt NK cell effector functions. The harsh TME owing to the nutrient deficiency, hypoxia, and acidic conditions can further suppress and dampen NK activities.
Future perspectives:
Paradigm shifting CAR-T-cell therapy has pioneered the development of the CAR technique and yielded promising outcomes in treating hematological tumors.
Benefiting from the technologies and valuable lessons learned from CAR-T cell therapy, CAR-NK cell therapy has advanced rapidly with continuous innovations.
Preclinical and early clinical outcomes have demonstrated the vast potential of CARNK cells as ‘off-the-shelf’ products for cancer treatment. To date, numerous strategies have been applied in CAR-NK cell therapies to address the challenges discussed above, and satisfactory outcomes have been observed in preclinical studies.
However, some of these strategies are difficult to translate into clinically approved procedures, such as the systematic infusion of NK-cell-stimulating cytokines. In contrast, multiplexed CAR-NK cell design systems or combinatorial approaches based on radiotherapy and other FDA-approved drugs may hold great potential to overcome the barriers in the CAR-NK cell therapy field and provide clinical benefit.
With the development of cutting-edge technologies such as single-cell RNA sequence analysis (scRNA-seq), we have access to elucidating key parameters associated with CAR-NK cell biological function and therapeutic efficacy, which may provide investigators and clinicians with critical insights into how to optimize the promise of NK cell-based cancer therapy.
In the coming years, clinical translation-oriented research and in-depth clinical testing of CAR-NK cell therapies are urgently needed to determine their potential market authorization.”
Source: Alexander González/LinkedIn


