Vivek Subbiah recently shared on his X/Twitter:
”First time disclosure of structure RevMed KRASG12D(ON) covalent inhibitor at AACR24.
- KRASG12D is the most frequent KRAS mutation in human cancers.
- What is the mechanism? RMC-9805 = first-in-class, oral, mutant-selective covalent inhibitor of the GTP-bound and active RAS(ON) form of KRASG12D.
- The formation of a stable, high-affinity tri-complex between RMC-9805, KRASG12D, and cyclophilin A.
Results in the suppression of signaling downstream of KRASG12D(ON) by disrupting its interactions with downstream effectors such as RAF kinases.”
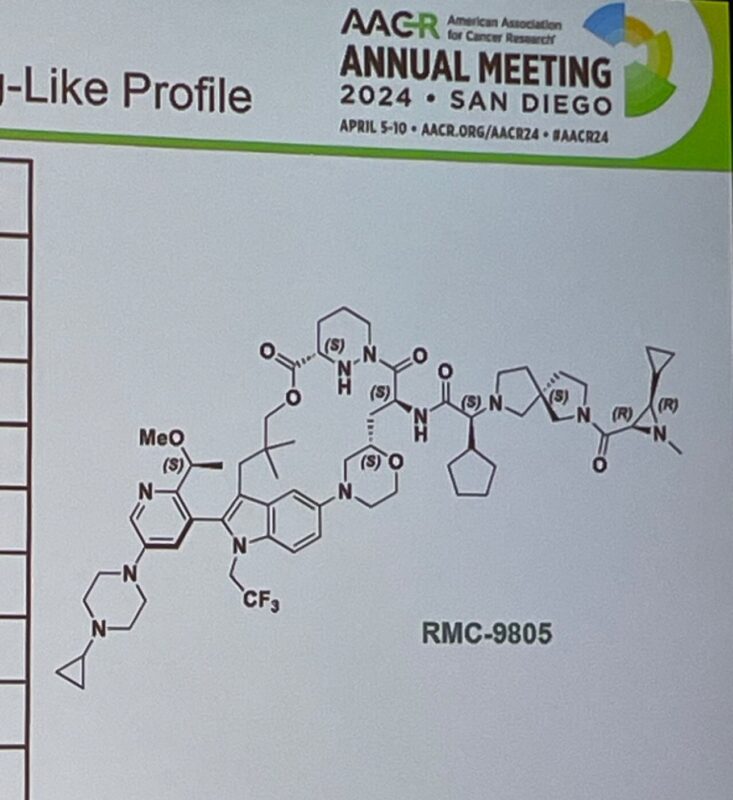
Source: Vivek Subbiah/X
Vivek Subbiah is the Chief of Early-Phase Drug Development at the Sarah Cannon Research Institute (USA). He is the former Executive Director of Oncology Research at the MD Anderson Cancer Network and a former Associate Professor in the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center. Dr. Vivek Subbiah has served as the principal investigator in over 100 phase I/II trials and co-investigator in over 200 clinical trials and is known for his leadership in several first-in-human and practice-changing studies that directly led to approvals from the FDA, European Medicines Agency, and other agencies across the world. He is an expert in tumor agnostic precision oncology and leads the BRAF and RET tissue agnostic studies to FDA approval.


