Talha Badar, Assistant Professor of Oncology at Mayo Clinic Comprehensive Cancer Center, posted on X/Twitter:
“ASH23 Highlights for me.

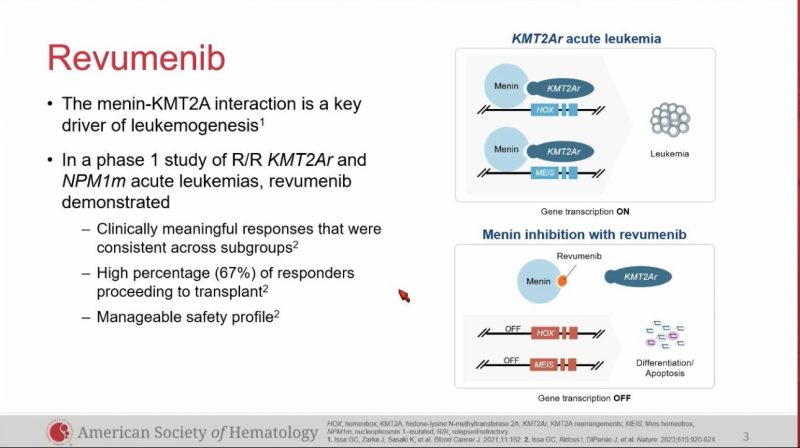
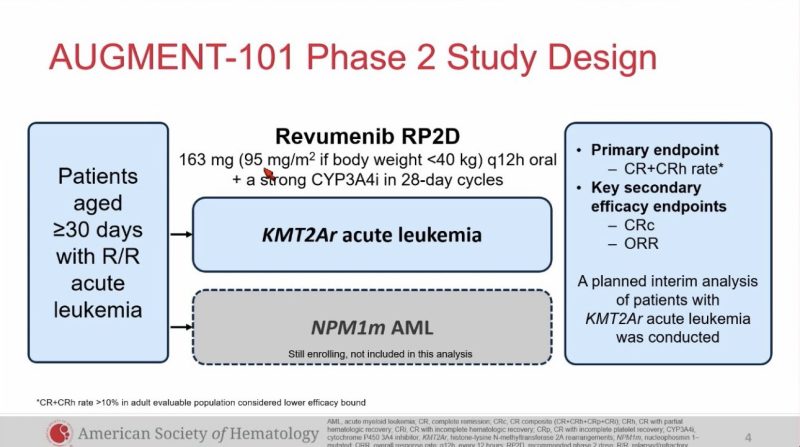
LBA: Augment-101 trial of revumenib in pediatric and adult R/R KMT2Ar acute leukemia.
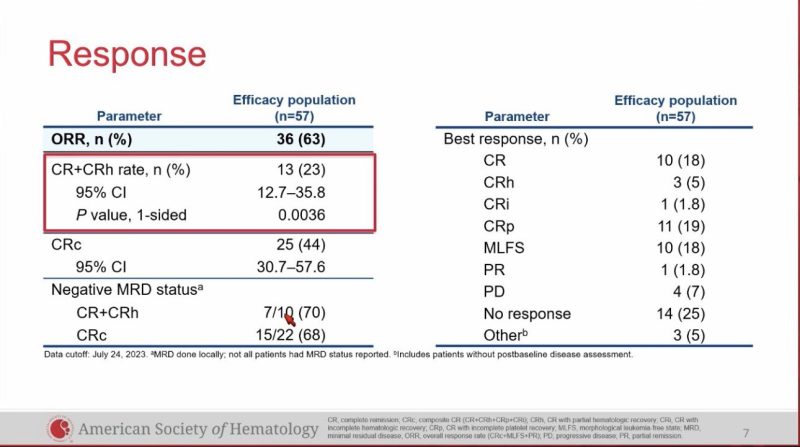
57 evaluable patients, ORR 63%, CR/CRh 23%, CRc 44%.
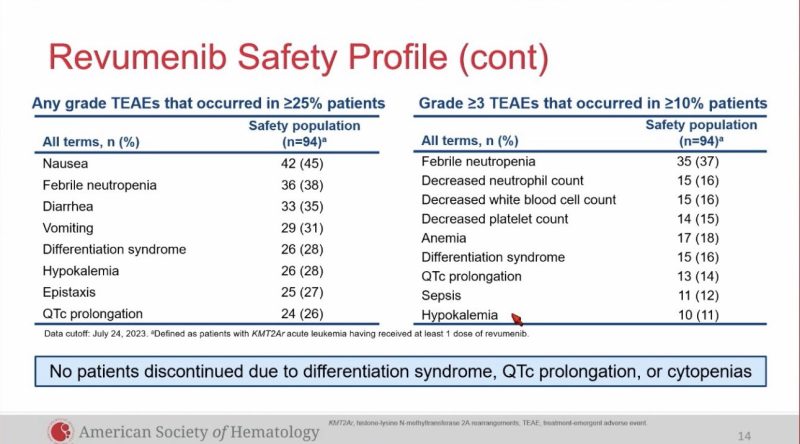
AEs: DS 28%, QTc prolongation 26%, did not let to study discontinuation.
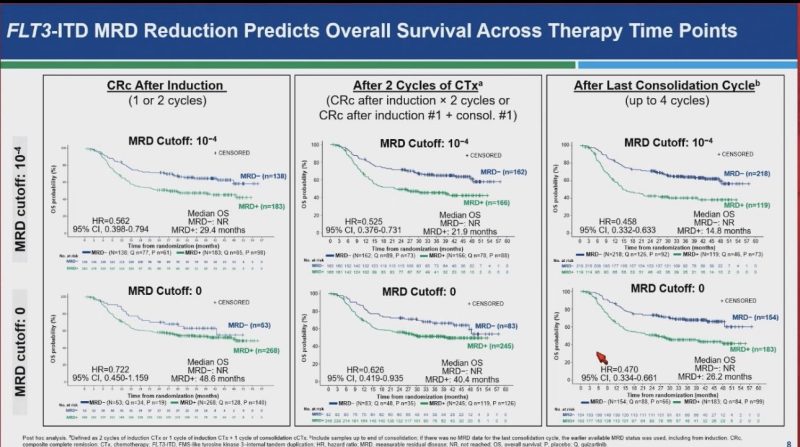
Paper 832: QuANTUM trial: FLT3-ITD-specific MRD demonstrates potential prognostic utility.
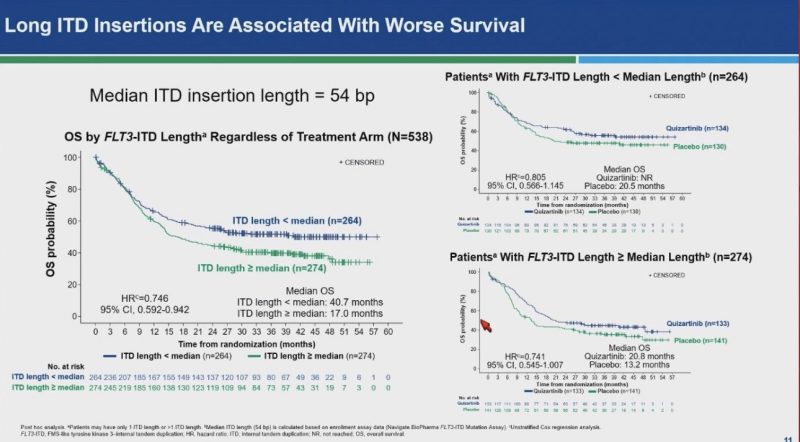
Long ITD insertion is associated with inferior outcome.
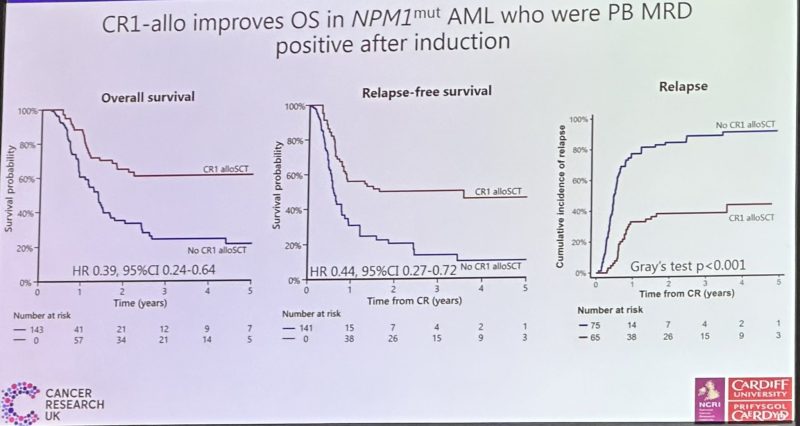
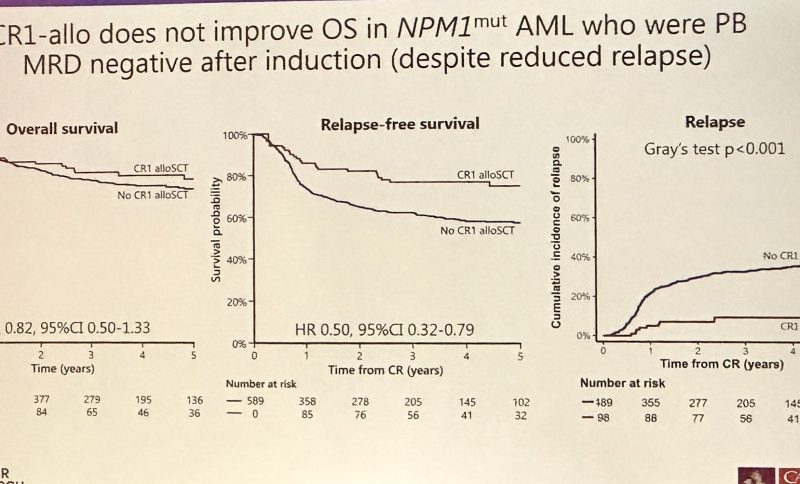
The Benefit of Allogeneic Transplant in 1st CR in NPM1m AML with or without FLT3 ITD Is Restricted to that Testing MRD plus after Induction – an Analysis of the UK NCRI AML17 and AML19 Studies
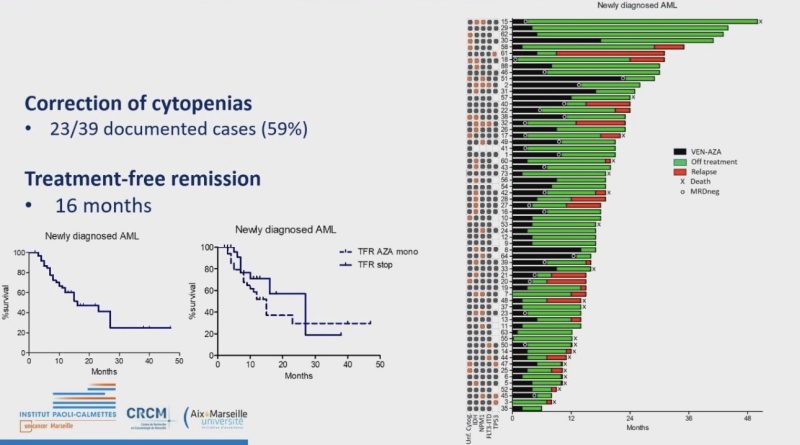
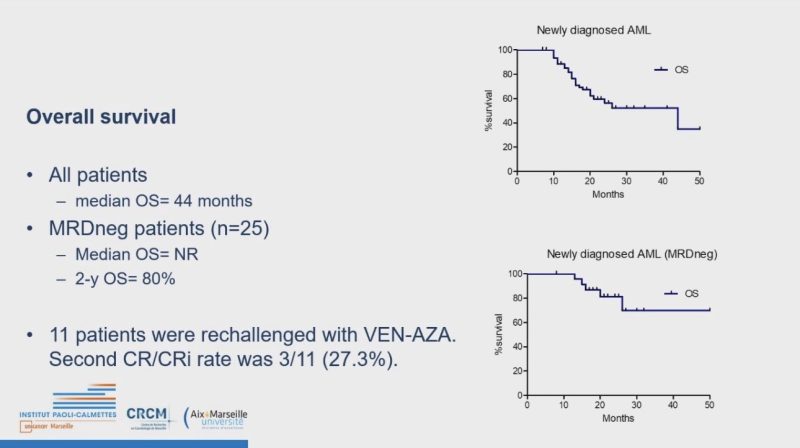
AMP patients Who Stopped Venetoclax or/and Azacytidine for reasons other than Progression Have a Prolonged Treatment Free Remission and Overall Survival: FILO
Minimum Rx 1 yr, MRD -ve before discontinuation: TFR 16 months.
Response rate ~ 40% on re-challenge.
TFR is higher in TN.

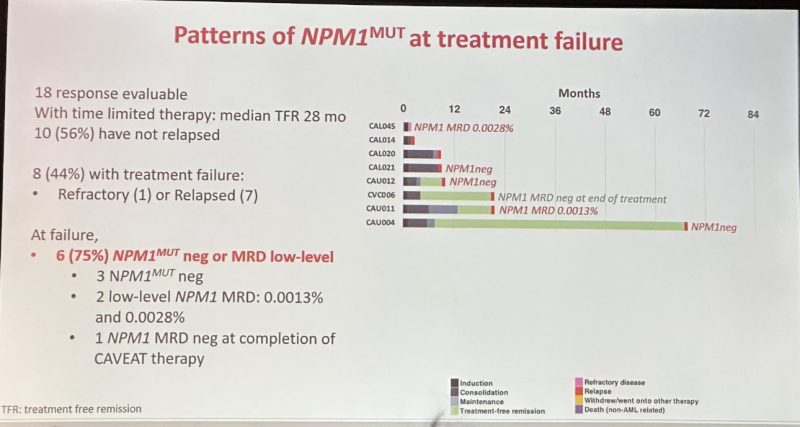
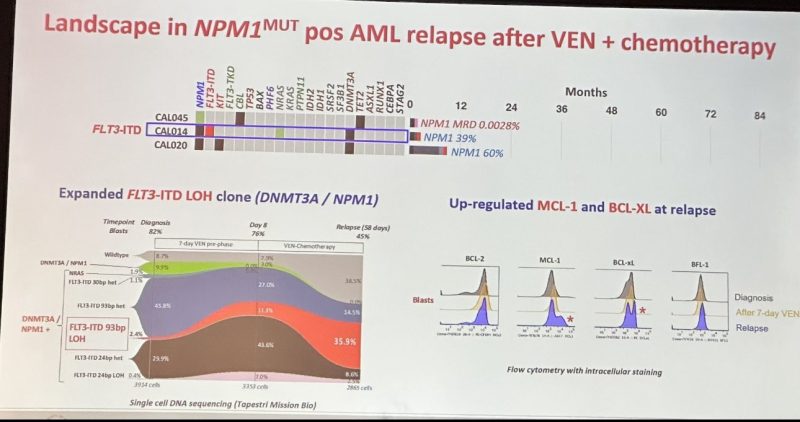
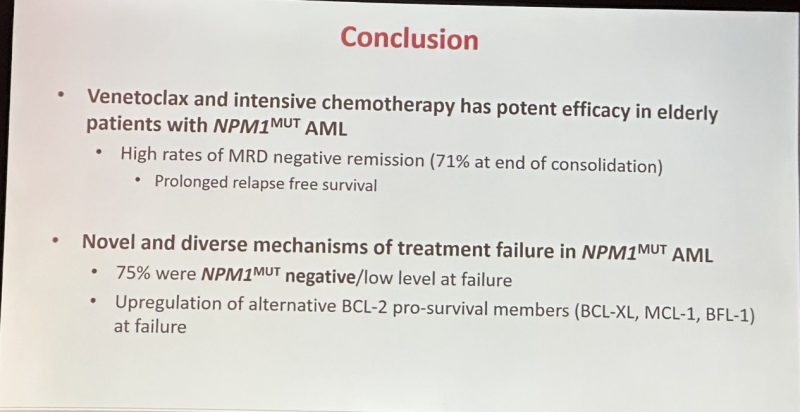
VEN Has Potent Efficacy in NPM1m AML with Acquired Resistance Associated with Perturbed Pro-Survival Signalling or NPM1 wt
Wonderful presentation utilizing single cell seq Identifying mech of relapse.
75% were NPM1 neg increased FLT3 LOH and BCL2 pro-survival members leads to relapse.
Phase 1b study:
-107 pt received R2PD dose of 14d ven, 42 went to allo
-30% CR rate
-mOS of 26 months (not censored for all)

Olverembatinib and venetoclax with reduced-intensity chemotherapy. All pts achieved CR at the end of cycle 1 and no tumor lysis syndrome or treatment-related deaths occurred.
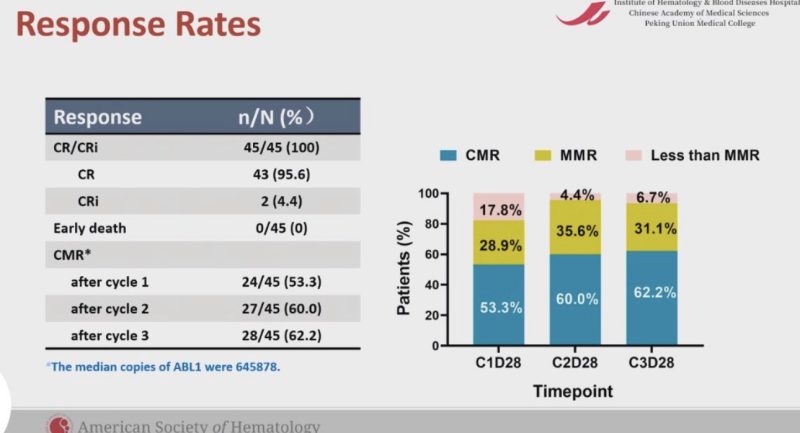
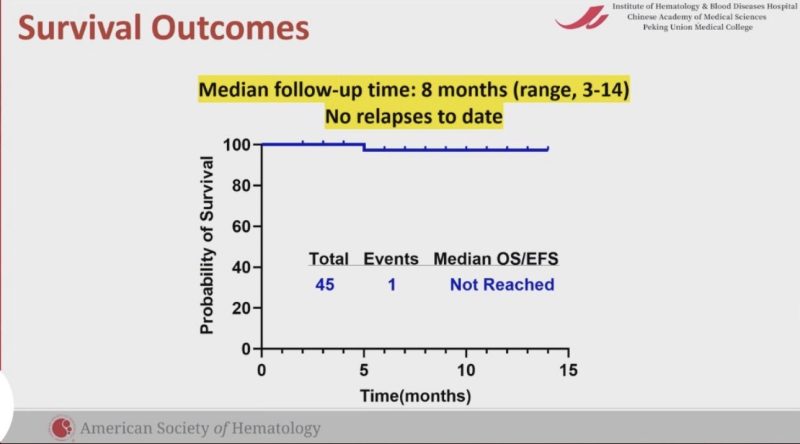
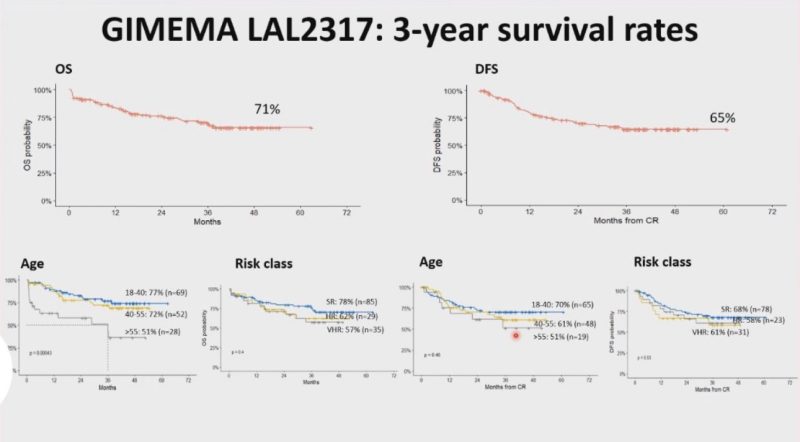
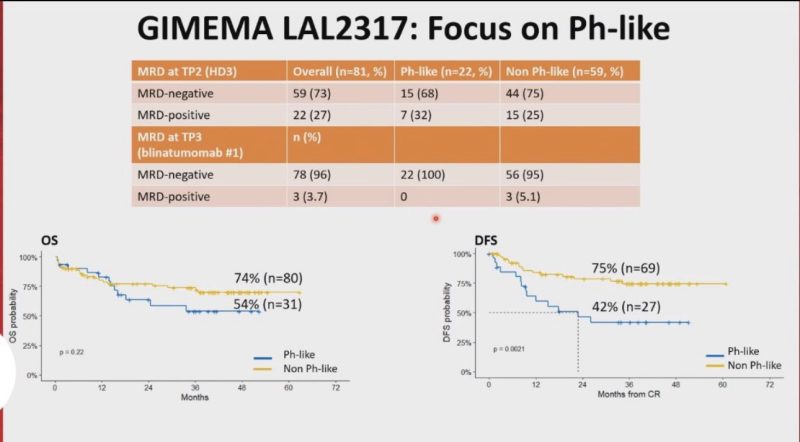
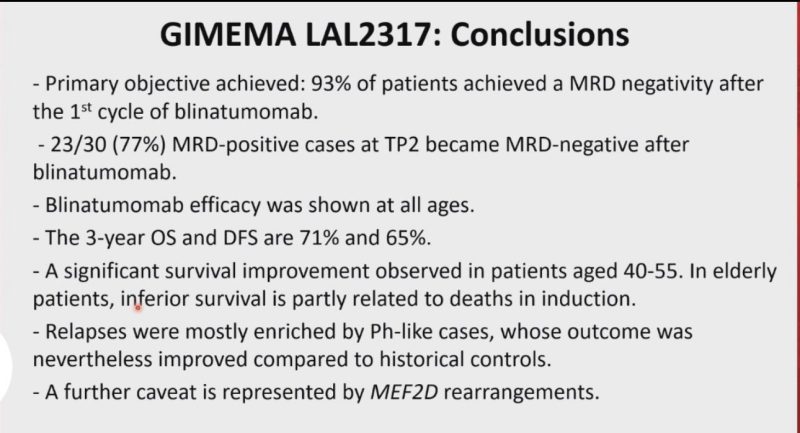
Phase 2 GIMEMA LAL2317 trial. Sequential chemo and Blina upfront!
OS and DFS were 71% and 65%, respectively. 93% of patients were MRD negative after the 1st cycle of blinatumomab!
869 Olverembatinib (HQP1351) Demonstrates Efficacy Vs. Best Available Therapy in Patients with Tyrosine Kinase Inhibitor (TKI)-Resistant Chronic Myeloid Leukemia Chronic-Phase (CML-CP) in a Registrational Randomized Phase 2 Study
3rd generation TKI, responses in ponatinib resistant patients.
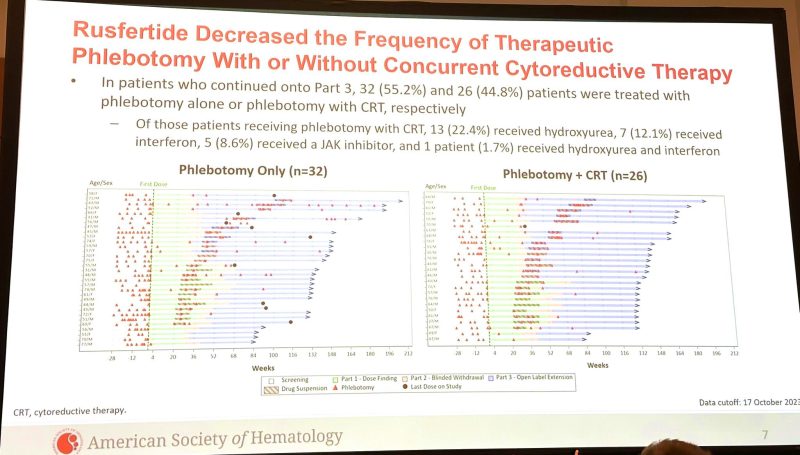
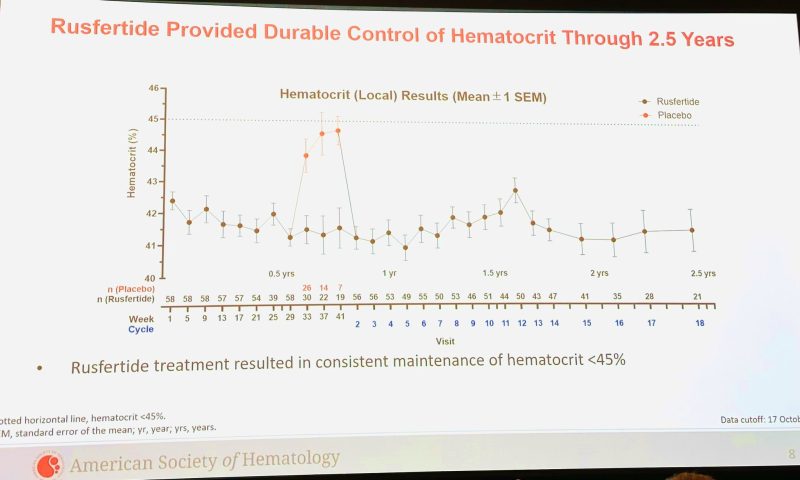
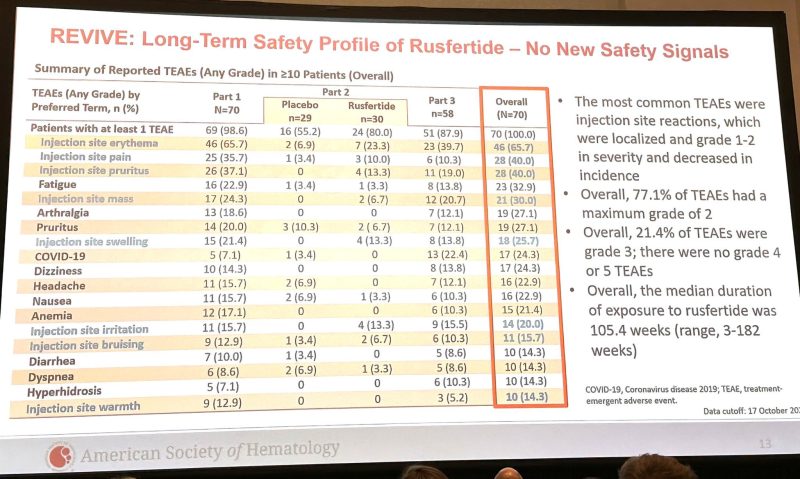
2-yr follow-up of REVIVE phase 2 hepcidin mimetic Rusfertide PTG300 | Results & conclusions:

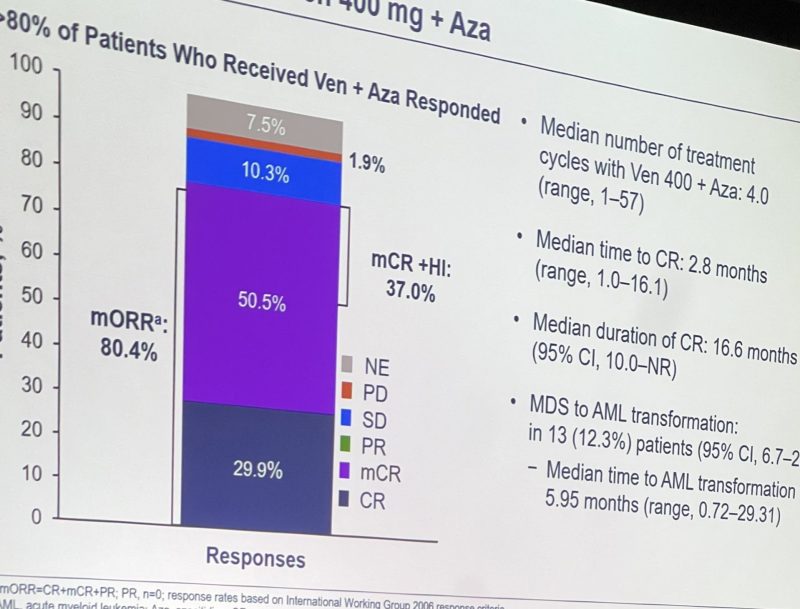
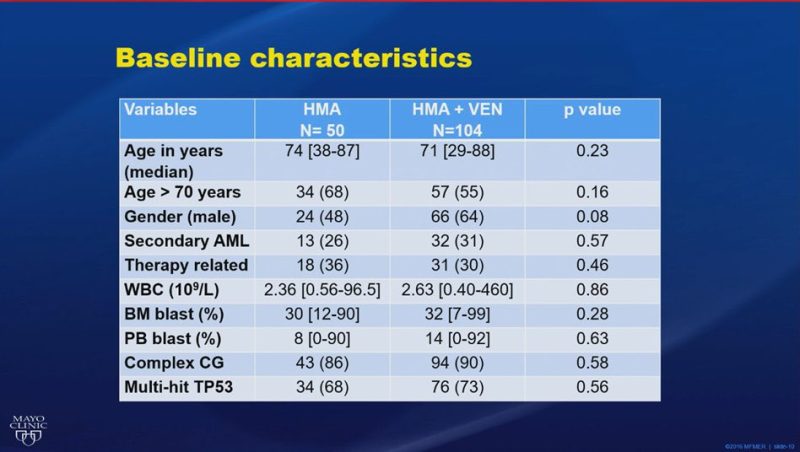
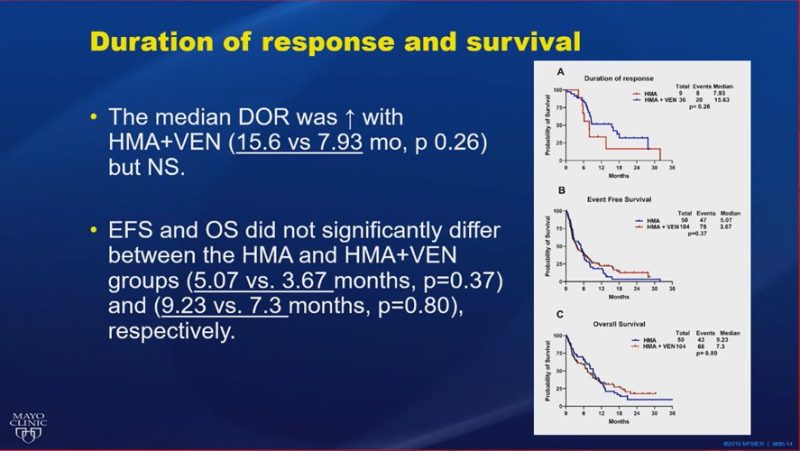
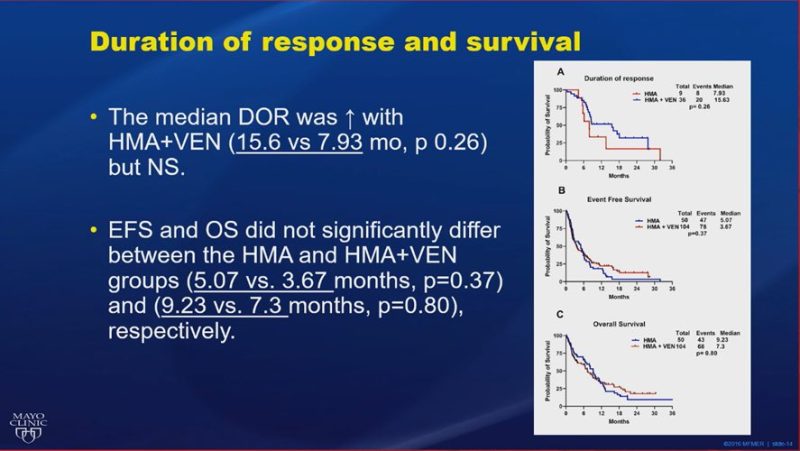
WD treatment-naïve TP53-mutated #AML treated with HMA+Ven (n=104) vs HMA (n=50). Improved CR/CRi rates (35% vs 18%), DOR (15.6 vs 7.93 months), and bridging to transplant (13% vs 4%), but no significant improvement in EFS or OS with HMA+Ven.”

Source: Talha Badar/X


