Vinay Prasad, Professor in the Department of Epidemiology and Biostatistics at the University of California San Francisco, made the following post on X/Twitter:
“Dave A. is a M4 at Icahn School of Medicine at Mount Sinai. He came to UCSF Epidemiology and Biostatistics in October. We wrote this paper then, which appears now in JAMA. Its about a wild drug approval Zuranalone for postpartum depression and interested students can rotate here with my email.

This is a new, oral drug approved for Postpartum depression that is expected to cost 30,000 It modulates GABA, similar to barbituates!

Blackbox warning is that it can hit you hard! Very hard. Somnolence, dizziness, sedation.

You can’t breastfeed on this drug You can’t get pregnant (again) on this drug Wowzers! a postpartum depression drug not compatible with breast feeding.

The trials that led to approval took SEVERELY DEPRESSED women and WITHHELD the standard of care of SSRI and counseling and instead gave them PLACEBO This is a huge concern. This was a harm to participants in control group, and problematic in our opinion.

Although the trials restricted to SEVERELY depressed woman, the FDA expanded the label to ALL post partum depression Not appropriate extrapolation.

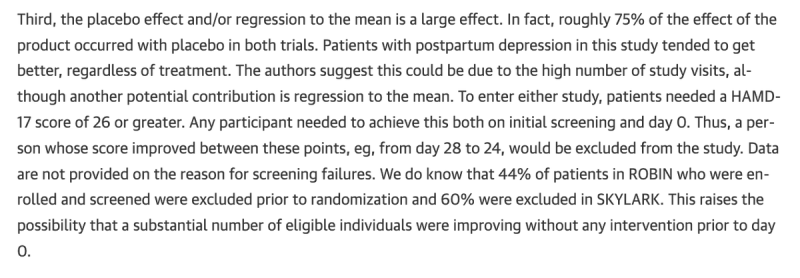
Third, there is a huge regression to the mean effect The actual effect size is slight.
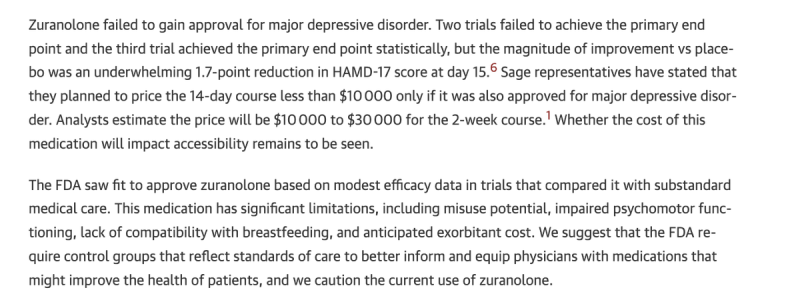
Finally the drug failed to improve outcomes in MDD.
All credit should go to Dave who brought the drug to my attention and quickly deduced the salient concerns. He writes the substack Dave the Knave and is applying for Anesthesiology residency now. He did this paper in 1 month. You should rank him highly.”

Source: Vinay Prasad/X


