Vincent Rajkumar posted the following on X:
“I estimate over 100,000 person years of life saved in myeloma due to the FDA accelerated approval pathway, without factoring recent approval of 3 bispecifics.”
Quoting Vincent Rajkumar’s earlier post:
“So far in myeloma we have got it right. Most drugs granted accelerated approval have proven to be effective in phase III.
Three were withdrawn. One didn’t do the phase III. One failed on phase III. One failed on first phase III but has two positive phase IIIs and will likely be back.”
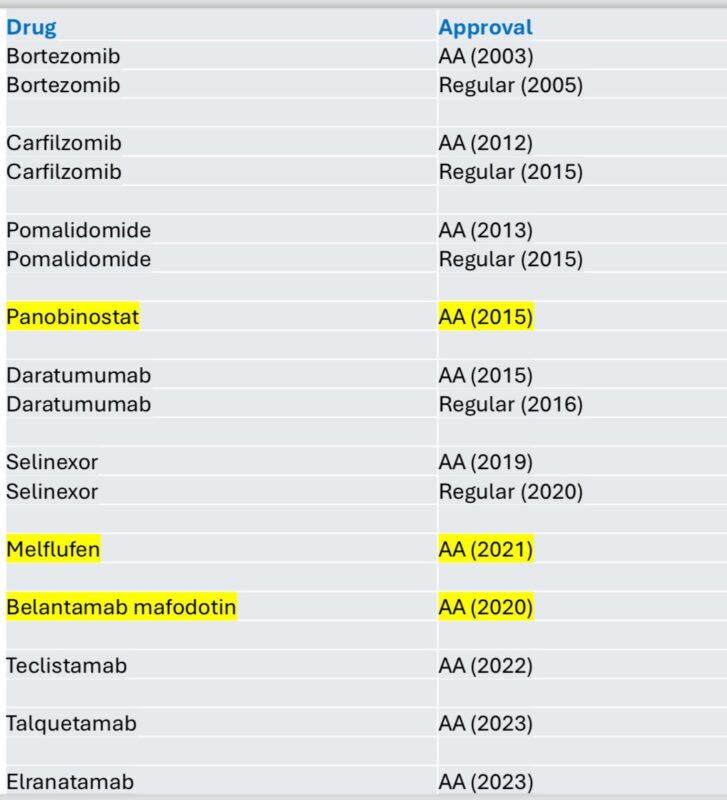
Source: Vincent Rajkumar/X
Vincent Rajkumar is a Professor of Medicine at the Mayo Clinic in Rochester, Minnesota, and Chair for the Mayo Clinic Myeloma, Amyloidosis, and Dysproteinemia Group. He also chairs the Board of directors of The International Myeloma Foundation and the Eastern Cooperative Oncology Group (ECOG) Myeloma Committee. His extensive contributions include over 230 peer-reviewed publications, predominantly focusing on multiple myeloma and related plasma cell disorders. Furthermore, Dr. Rajkumar is a Section Editor for multiple myeloma and related disorders for Leukemia and an Associate Editor for the Mayo Clinic Proceedings.


