Toni Choueiri shared a post on X:
“Results from TiNivo2 are out both ESMO24 and The Lancet! Another phase 3 trial to assess the efficacy of immunotherapy rechallenge, comparing tivozanib + nivolumab vs. tivozanib monotherapy in patients with RCC following an immune checkpoint inhibitor (ICI). (link)
Prior tivozanib trial (TIVO-3) showed an improvement in progression free survival (PFS) with tivozanib compared with sorafenib (HR = 0.55; p=0.028) in the subgroup (26%) that received previous ICI. (link) 
CONTACT-03 trial (atezo + cabo to cabo-monotherapy), the first randomized phase 3 evidence of ICI rechallenge in mRCC, revealed negative results, suggesting that ICI rechallenge should be discouraged in patient with mRCC (link)

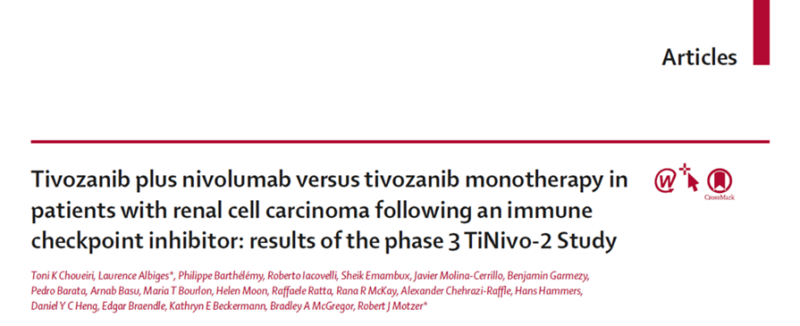
TiNivo-2 is multicenter, randomized, open-label, phase 3 trial
Inclusion criteria were:
- Metastatic ccRCC
- ECOG PS: 0 or 1
- Prior progression on 1-2 regimens (one ICI), following ≥ 6 weeks of ICI
Time from prior line discontinuation to randomization ≤ 6 months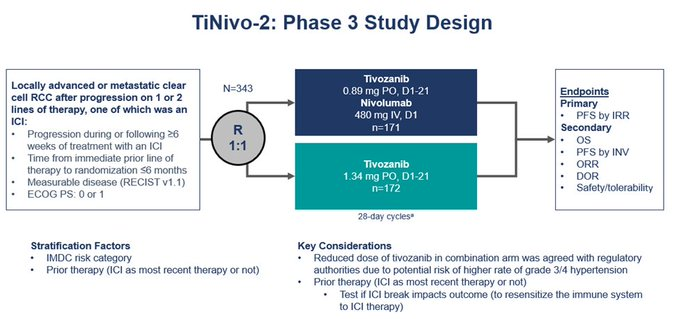
Patients were randomized 1:1 to tivozanib (0.89 mg/day, orally) + nivolumab (480 mg every 4 weeks, IV) or tivozanib (1.34 mg/day, orally)
1ary endpoint: PFS (central review)
2ary endpoint: OS/ORR
343 patient were assigned to tivozanib-nivolumab (171) or tivozanib monotherapy (172).
Time from randomization to data cutoff was 12.0 months.
Baseline characteristics:
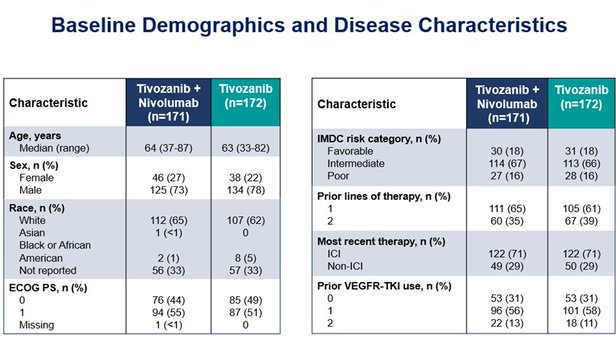
No PFS benefit was observed with Tivo+Nivo vs Tivo only
At 12 months of follow-up, PFS was 5.7 months (95% CI 4.0–7.4) in the Tivo+Nivo group and 7.4 months (5.6–9.2) in
Tivo-monotherapy group (HR = 1.10 (95% CI 0.84–1.43; p=0.49)).
Predefined analyzes per strata were consistent with the primary analysis, whether patients had received an ICI as part of their most recent therapy or not, and whether the study drug was 2nd or 3rdline therapy.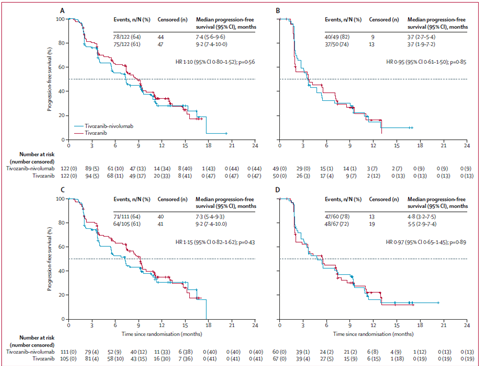
Median OS: 17.7 m (95% CI 15.1–not reached [NR]) in the Tivo+Nivo group vs. 22.1 m (15.2–NR) in the Tivo-monotherapy group (HR:1.0).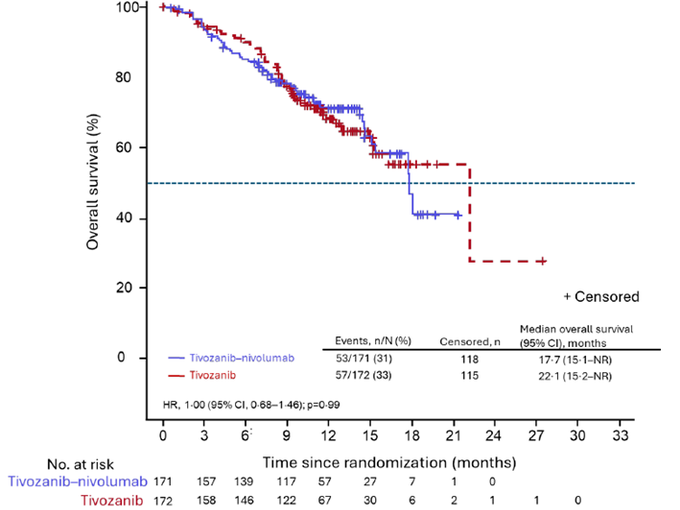
ORR: 19% (95% CI 13.7-26.0) in the Tivo+Nivo group vs. 20% (14.1–26.5) in the Tivo-monotherapy group.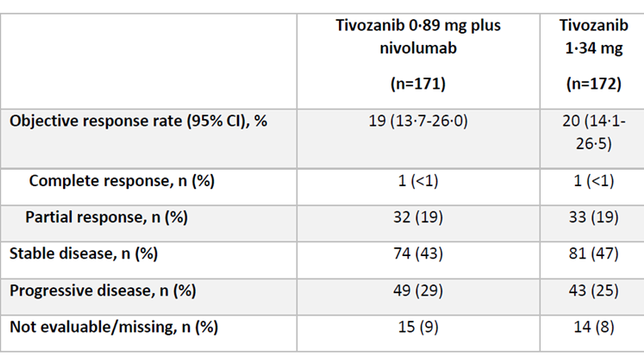
Grade 3+ adverse events (AEs) were ~60% in both treatment arms.
Most common grade 3+ AEs was hypertension, reported equally in both groups
at 75 (22%).
AEs led to withdrawal in 16% and 19% of pts on Tivo+Nivo and Tivo-montherapy, respectively.
In conclusion, the addition of PD-1 inhibitor nivolumab to tivozanib did not result in improved clinical outcomes in patients with mRCC whose disease progressed on or after prior ICI treatment. This trial confirms and expands key conclusions from CONTACT-03 and suggests that ICI rechallenge should be generally discouraged regardless of treatment sequence.
Huge thanks to all the investigators who made this trial possible, to AVEOOncology, the sponsor, and mostly to our patients and their families, to whom we dedicate all our efforts!”
Source: Toni Choueiri/X
Toni K. Choueiri is the Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute (DFCI), co-leader of the Kidney Cancer Program at Dana-Farber/Harvard Cancer Center, and the Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School. As a medical oncologist, clinical trialist, and translational researcher, he specializes in treating genitourinary cancers (prostate, bladder, testis, and kidney cancer), with a focus on kidney cancer.
More posts featuring Toni Choueiri on oncodaily.com


