The 2025 Society for Neuro-Oncology (SNO) Annual Meeting is taking place from November 19th to 23rd in Honolulu. The event is bringing together global leaders in neuro-oncology to share new research, clinical insights, and emerging therapeutic innovations for brain and CNS tumors. This year’s theme, ‘30 Years of Progress,’ highlights the scientific and clinical breakthroughs that have shaped the field since SNO’s founding in 1995.
Elias Ortega Chahla, Medical Director (Hematology/Oncology), IQVIA, shared insights from SNO 2025 Conference on LinkedIn:
The Emergence of General AI for Biomedicine
“One of the most compelling sessions at this year’s meeting showcased how AI is evolving from standalone models into orchestrated ecosystems that meaningfully support clinical decision-making.
What stood out wasn’t just the technology—it was the shift in mindset:
From technology substitution → to ecosystem transformation
This isn’t about upgrading old tools. It’s about reshaping how clinicians think, collaborate, and create.
Agentic AI as a new paradigm
Human clinicians working with an intelligent orchestrator that coordinates specialized agents—radiology, pathology, staging, literature, clinical trials, documentation.
A digital architecture that mirrors a multidisciplinary tumor board, but works continuously and at scale.
Real clinical impact already happening:
• Generative AI producing notes, patient instructions, billing codes
• Sequential diagnostic frameworks (SDBench) exploring uncertainty
• GraphRAG enabling global biomedical sense-making
• GPT-powered structuring of real-world patient data
• AI-enhanced imaging reconstruction pipelines
The takeaway:
AI won’t replace clinicians – but clinicians who harness orchestrated AI systems will redefine what modern care looks like.”
Artificial Intelligence for Neuroimaging
“A fantastic session today diving deep into how AI and radiomics are reshaping neuro-oncology imaging.
What I appreciated most was how the talk connected fundamental concepts to real clinical impact:
AI, ML, Deep Learning — demystified
Clear distinctions between the layers of modern AI and how deep learning is driving image-based insights.
Radiomics: images as data
The late Robert Gillies’ message echoed throughout the session:
“Images are more than pictures—they are data.”
Radiomics builds on the hypothesis that imaging reflects underlying tumor morphology, and the workflow—from acquisition to segmentation to feature extraction—continues to mature.
Neuroimaging applications that matter:
• Differentiating post-treatment effects
• Predicting molecular biomarkers (radiogenomics)
• Tumor detection & segmentation
• Prognosis, therapy response & disease progression modeling
Realistic assessment of progress
Despite impressive academic success and early software tools, clinical translation remains challenging—underscoring the need for explainable AI, multicenter datasets, and transparent evaluation.
The closing message resonated strongly:
AI should be a partner in decision-making, not a competitor — “Human AND Machine.””
Artificial Intelligence for Pathology
“Another excellent session today, highlighting how AI is reshaping modern pathology—from routine workflows to deep biological insights.
This talk focused on three powerful applications:
- Automating traditional tasks
AI is increasingly capable of handling repetitive, labor-intensive steps in pathology—streamlining workflows without replacing expert judgment. - Understanding intra-tumoral heterogeneity
Deep learning–based feature extraction and clustering are now enabling spatial maps of histomic variation within tumors—revealing patterns that the eye simply can’t detect.
A fascinating look at how whole slide images can be transformed into rich, quantifiable biological landscapes. - Refined outcome prediction in gliomas
AI-augmented risk models integrating molecular subtypes, grading, and image-derived features showed striking improvements in survival stratification.
A glimpse at how AI could strengthen future WHO classification and personalize prognosis.
The overarching message:
AI isn’t just automating pathology—it’s expanding what pathology can see and predict.
And, importantly, these systems are being developed as collaborative tools, not competitors.”
AI for Spatial Imaging & Multimodal Data Fusion
“This session dug into one of the most exciting frontiers in neuro-oncology AI: bringing together high-dimensional, multimodal patient data to better understand tumors and predict outcomes.
- Why multimodal matters
Cancer patient data spans EHRs, radiology, pathology, and molecular sequencing—each with unique strengths and gaps. The session outlined how embedding frameworks now make it possible to integrate all of these data types into unified models. - Data fusion in action
Examples across adult and pediatric brain tumor cohorts showed how combining histopathology with RNA-seq significantly improves survival prediction compared to single-modality models. - Joint fusion architectures
A clear walkthrough showed how whole-slide pathology features and transcriptomic features can be fed into joint neural models—producing refined risk scores that outperform siloed approaches. - Transfer learning across tumor types
Models trained on adult gliomas can be fine-tuned for pediatric tumors (and vice versa), enabling knowledge sharing across rare cohorts and reducing data requirements.
Key takeaways:
• Multimodal fusion boosts predictive performance for survival, grading, and key biomarkers (IDH1, MGMT).
• Spatial models can map biologically meaningful features directly onto H&E slides.
• Synthetic FFPE tiles (RNA-CDM) can preserve cellular proportions—opening doors for training foundation models.
• Spatial omics is now feasible on small cohorts, with AI bridging gaps between imaging and molecular signatures.
• The future points toward multimodal modeling + foundation models for unified cancer understanding.
This session made it clear how rapidly we’re moving toward spatially-aware, data-rich AI systems that can reveal biology we’ve never been able to see before.”
WHO Classification and cIMPACT-NOW: Where Are We Going?
“A highly informative session on the evolving landscape of CNS tumor diagnostics, highlighting how molecular profiling and methylation data continue to reshape modern classification.
- cIMPACT-NOW Update 9: Key recommendations
• DNA methylation profiling is now strongly endorsed as a valuable diagnostic tool across CNS tumors.
• Results should always be interpreted within the full clinical and pathological context.
• A layered diagnostic format incorporating methylation profiles is recommended for clarity and consistency. - Refinements in diagnostic criteria
Updates across major entities—GBM (IDH-wildtype), diffuse pediatric-type high-grade gliomas, and posterior fossa ependymomas—highlighted the shift toward:
• Integrating methylation class assignments
• Recognizing the limits of single-marker interpretation
• Using molecular surrogates when methylation is not available - Posterior fossa ependymoma updates
Diagnostic clarity continues to improve with features such as:
• Loss or retention of H3K27me3
• Presence/absence of EZHIP expression
• Copy number stability and genome-wide patterns
Clear take-home points emphasized how IHC and molecular tools complement each other. - What’s next: cIMPACT-NOW 12 & 13 and CNS6 (likely “WHO 2027”)
Upcoming initiatives will focus on:
• Genetic tumor syndromes from a practical pathology perspective
• Molecular grading refinements for IDH-mutant astrocytomas
• Expanding guidelines to better support LMICs where resources are limited
The session underscored how rapidly the field is moving toward integrated, molecularly grounded standards, balancing precision with real-world feasibility.”
Biomarkers for Early Detection & Monitoring of Brain Tumours
“A powerful session highlighting how liquid biopsy is transforming the way we diagnose, stratify, and follow brain tumours – especially in pediatrics.
- Why liquid biopsy matters
Liquid biopsy enables detection of tumour-derived DNA/RNA/proteins from fluids such as CSF, blood, saliva, urine, and effusions. Its sensitivity depends on tumour location, burden, stage, analyte type, and test platform. - Clinical applications expanding rapidly
• Early detection and screening for high-risk groups
• Diagnosing tumours that are difficult or risky to biopsy
• Treatment selection via mutation and biomarker profiling
• Monitoring therapy response with real-time molecular readouts
• Tracking tumour evolution and emerging resistance mutations
• Detecting minimal residual disease (MRD) after therapy
• Identifying early relapse before radiographic progression - Evidence across tumour types
Baseline ctDNA positivity varies greatly:
• High-grade glioma: 77%
• Low-grade glioma: 27%
• Germ cell tumours: 93%
• Medulloblastoma: 54%
Importantly, ctDNA status strongly predicts survival, underscoring its value as a prognostic biomarker.
Key takeaways
• Liquid biopsy shows tremendous promise for non-invasive diagnosis, especially for LGG, HGG/DMG, and germinomas.
• MRD assessment is emerging as a critical tool for medulloblastoma, embryonal tumours, ependymoma, MMRD tumours, and HGG.
• Lower morbidity and cost compared to surgical biopsy.
• ctDNA remains the most widely used analyte today, but advances in WGS and multi-omic profiling are rapidly expanding capabilities.
This session made it clear: liquid biopsy is poised to become a central pillar of precision neuro-oncology, enabling earlier intervention, better risk stratification, and smarter treatment decisions.”
30 Years of Therapy for Adult Glioma (Susan Chang)
“A powerful look at how neuro-oncology has transformed – from limited imaging and no molecular diagnostics in 1995 to today’s integrated, technology-driven care.
- Milestones in LGG & GBM
• PCV, temozolomide, and IDH mutation reshaping classification
• TTFields, BRAF-targeted therapy, Vorasidenib
• Practice-changing trials from EORTC, RTOG, CATNON
- Core Themes Driving Progress:
A. Standardization – RANO evolution, PET integration, global harmonization
B. Technology – Molecular diagnostics, advanced imaging, AI for segmentation, grading, and intra-op support
C. Translational Bridges – Clinical observations informing science; molecular insights guiding therapy; correlative studies embedded in trials
D. Collaboration – Multidisciplinary care, cooperative trials, international standards
- Neuroimaging Advances
Better tumor delineation, guidance for surgery, physiologic/metabolic imaging to detect progression, and standardized response assessment. - Holistic Patient-Centered Care
UCSF integrates clinical care, caregiver support, survivorship, neurocognitive services, and community outreach. - Looking Forward
Progress over 30 years shows the value of standardization, technology, translational research, and global teamwork—the same pillars that will guide the next decades.”
Integrating IDH Inhibitor Therapy Into Glioma Care
“A forward-looking session underscoring how IDH inhibitors are reshaping the treatment landscape for IDHm gliomas:
New targeted option:
IDH inhibitors introduce a novel class of oral therapies for IDH1/2-mutant gliomas.
Phase 3 success:
Trials show significant PFS improvement vs. placebo in Grade 2 IDHm gliomas – a major milestone in early-stage disease.
Impact on tumor biology:
Data suggest IDH inhibitors slow tumor growth and delay progression, opening doors to earlier, less-toxic intervention strategies.
Favorable safety:
Consistently manageable AE profiles – most adverse events are mild to moderate.
A shifting paradigm:
IDH inhibition marks a new era in glioma care, offering hope for patients who previously had limited options.”
Reimagining Clinical Trials in Glioblastoma
“Clinical trials for GBM are shifting from static protocols to adaptive learning, where every step becomes a discovery experiment. This approach links drug exposure, biology, and real-time patient response to guide smarter decisions. Instead of asking “Does it work?”, the new focus is “How — and for whom — does it work?”
Each patient contributes valuable insight through integrated PK/PD, genomic, and immune profiling. With continuous testing → learning → refining, progress moves beyond incremental change.
The future of GBM research isn’t about bigger trials — it’s about faster, smarter, biologically informed ones, where every dataset helps drive the next breakthrough.”
Keynote by Adrienne Boire, MD, PhD
“Leptomeningeal Metastasis: Unlocking the Biology Behind One of Neuro-Oncology’s Toughest Challenges
What an incredible plenary keynote — Dr. Adrienne Boire delivered a powerful and deeply insightful overview of leptomeningeal metastasis (LM), highlighting how biology-driven innovation is reshaping hope for these patients.
Understanding LM
• Cancer spreading into the leptomeninges of the brain & spine
• Primary sources: lung, breast, melanoma
• Outcomes remain limited → urgent need for better approaches
Liquid Biopsy as a Discovery Engine
CSF + blood samples become real-time biological sensors:
• RNA sequencing
• cfDNA exome analysis
• Proteomics + metabolomics
• Computational biology to map pathways & interactions
Every patient sample accelerates discovery.
The LM Microenvironment Matters
The leptomeninges actively shape tumor behavior:
• Inflammatory signaling at the choroid plexus
• Immune cell dynamics in CSF
• Microenvironmental cues supporting tumor growth
A deeper understanding = smarter targeting.
From Patient → Mechanism → Therapy
• Patient samples inform lab models
• Mechanistic pathways are mapped
• New therapeutic hypotheses emerge
• Precision-designed strategies follow
The future is smarter, faster, biology-first.”

“In a packed plenary session, Dr. Heiland dove into how data-driven neurosurgery is reshaping every step of brain tumor care — from the OR to molecular profiling to AI-powered interpretation. A masterclass in how technology is becoming inseparable from neuro-oncology.
Key Takeaways:
- The Data Explosion
From Cushing’s early surgical innovations → imaging revolutions → today’s multimodal, real-time data streams. We’ve entered a true data-driven era. - Integrated Neurosurgical Decision-Making
The OR of the future is a data hub:
• Pre-op MRI + functional mapping
• Intra-op rapid sequencing, fluorescence, navigation
• Post-op molecular review → therapy selection - High-Neural Signature Matters
Tumors with a high-neural ecosystem show significantly worse survival — underscoring the need for biology-aware surgical strategies. - Explainable AI in the OR
AI uncovers microglial–astrocytic reactive compartments and predicts tumor “neural score,” bringing unprecedented clarity to tumor biology in real time. - Resect 2 Disconnect
High-neural tumors benefit most from a larger extent of resection — shifting surgical philosophy toward biologically informed goals.
A brilliant look at the convergence of surgery, molecular science, computational biology, and AI. The future of neuro-oncology is integrated, intelligent, and intraoperative.”

Tailored Trials for Rare Tumors: Innovative Approaches to Study Design & Implementation
“A fantastic deep-dive into how rare tumor research is evolving — and why traditional models simply don’t fit. Dr. Robison highlighted the importance of rethinking trial strategy from the ground up, with patients and advocates leading the way.
Key Takeaways
• The 20th century model of “universal cancer biology” no longer applies — molecular complexity has reshaped the landscape.
• Rare cancers historically operated in a “regulatory free zone,” but today’s therapies demand clarity, rigor, and precision.
• Molecular classification has exploded, fragmenting what used to be single disease categories (e.g., medulloblastoma → multiple molecular subtypes).
• In the last decade, targeted therapies have accelerated rapidly — a steep growth curve marked by approvals from 2015 → 2025.
• Patients at the center — involvement must go beyond listening. True partnership means letting patients define the problems we aim to solve.
• No trial wasted — even negative trials should generate biologically meaningful insights through clear endpoints and phase-0/surgical arms.
• Global collaboration is essential to prevent duplicative efforts and prioritize the most impactful studies.
• The future requires adaptive endpoints, shared decision-making, and coordinated international effort.
Why it matters
Progress in rare tumors depends on smarter trial design, deeper patient engagement, and agreement across borders. There’s no “one size fits all” — but there is a path forward built on precision, partnership, and global alignment.”
Reimagining Rare Tumor Trials: AI, Remote Monitoring & the Decentralized Future
“In this forward-looking session, Dr. Shaalan Beg highlighted how rare-tumor research is being reshaped by AI, real-time digital tools, and decentralized clinical trial models—all aimed at accelerating discovery while reducing burden for patients.
Key takeaways:
- Decentralized endpoints are evolving—from home-replicable assessments (ECOG, RECIST) to novel digital biomarkers requiring new validation pathways.
- AI in clinical research is no longer theoretical—practical applications now span workflow automation, assay selection, quality control, and pathology review.
- Digital pathology promises faster triage, biomarker-negative identification, reduced burden, and expanded access, while still navigating regulatory acceptance and bias mitigation.
- Ambient listening technologies may transform AE reporting, monitoring, and documentation by turning natural clinical interactions into structured data.
- The future will demand true integration of digital tools into clinical context, workflow alignment, and robust data standardization to unlock the full potential of AI-enabled trial models.
The message was clear: to keep pace with the rising complexity of targeted therapies and delivery systems, the field must embrace smart automation, decentralization, and intelligent data systems—not as add-ons, but as core trial infrastructure.”
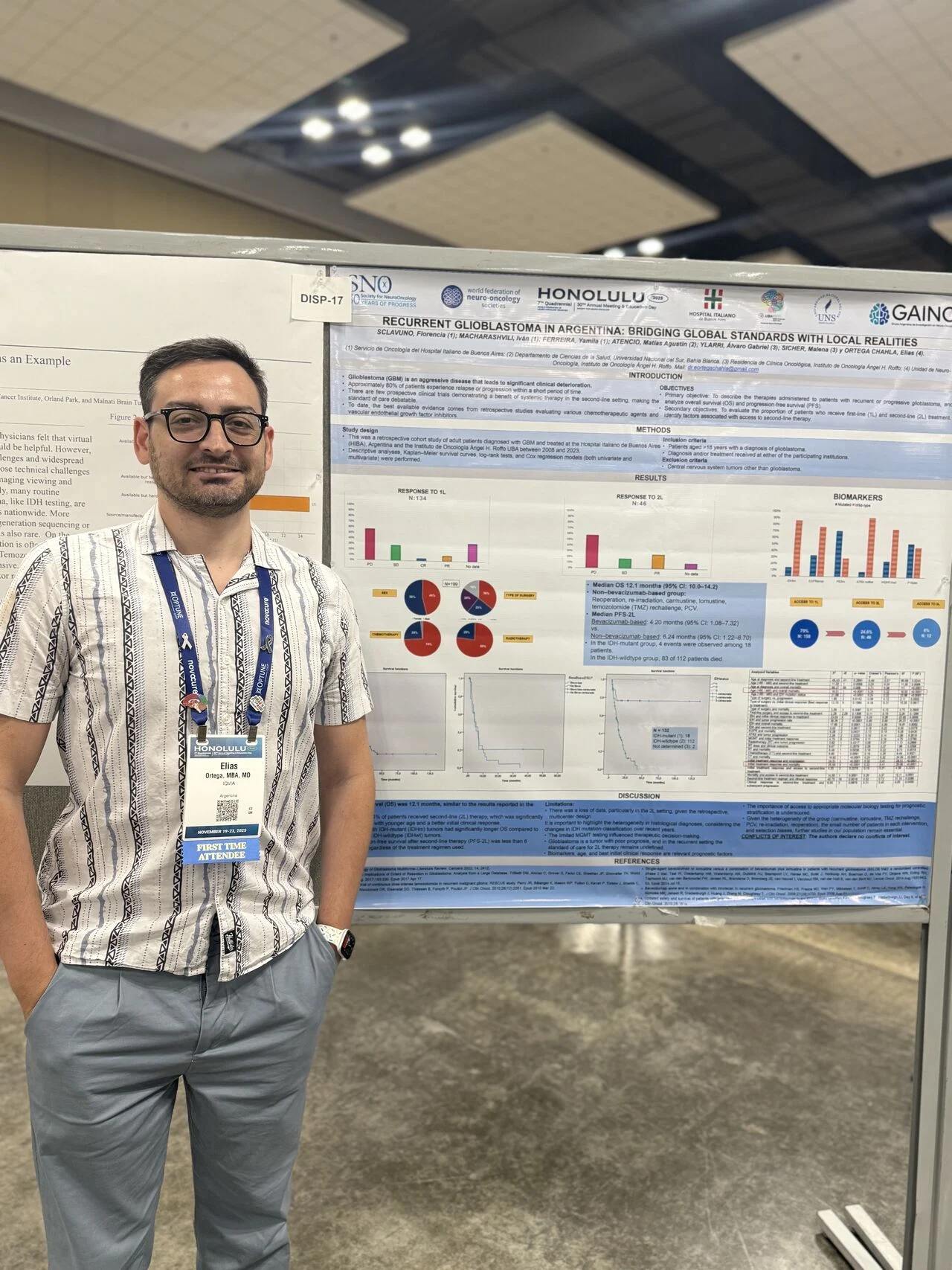
“Thrilled to share that our team presented two posters at SNO 2025 in Honolulu, Hawaii, showcasing the growing strength and commitment of Argentina and Latin America in neuro-oncology research.
1. Recurrent Glioblastoma in Argentina: Bridging Global Standards With Local Realities: Our multicenter retrospective study highlights how patients with recurrent GBM are managed in real-world Argentine institutions, emphasizing survival outcomes, access to second-line therapies, molecular testing challenges, and the urgent need for region-specific evidence.
2. Glioblastoma Treatment in Latin America: A Scoping Review: This work maps the regional landscape of GBM treatment across Latin America and the Caribbean, revealing fragmented evidence, methodological heterogeneity, and persistent inequities in access to therapies and data generation—while also identifying strengths and opportunities for collaborative progress.
Presenting these studies at SNO is more than academic visibility—it’s a reminder of why producing high-quality research from LATAM matters:
- Our region faces unique barriers and realities that must be understood to improve patient care.
- Strengthening local evidence is key to advancing precision medicine and informing public health strategies.
- Regional collaboration is essential to close gaps in access, diagnostics, and treatment innovation.
Special mention to Matías Agustín Atencio and Iván Macharashvili for being key contributors and authors of these abstracts.
Proud to contribute to the growing voice of LATAM in global neuro-oncology. Let’s keep building, researching, and pushing for equitable advances for all patients.”
Follow-Up/Subgroup Highlights: HA-WBRT vs SRS/SRT
“Study: Phase III Trial in Patients With 5–20 Brain Metastases
Key Results (Corrected):
SRS/SRT outperformed HA-WBRT in preserving cognition among 1-year survivors.
• Less cognitive decline across memory, processing speed & executive function
• Differences became most pronounced after 4 months
• Highlights that long-term cognitive outcomes matter more than ever as systemic therapies extend survival
Study Strengths:
• RCT design with predefined endpoints
• Longitudinal within-subject analyses to reduce attrition bias
Limitations:
• Smaller sample → limited ability for multivariable analysis
• <50% neurocognitive follow-up at 12 months
Future Questions:
• What patient-level factors predict decline or resilience?
• How do symptoms evolve in 12-month survivors?
• Need for neurocognitive follow-up beyond 12 months
Takeaway:
For patients with multiple brain metastases, SRS/SRT may offer a meaningful cognitive advantage over HA-WBRT – an important consideration as survival improves and quality of life becomes central.”
“Excited to start the SNO/WFNOS 2025 conference here in beautiful Honolulu, Hawaii.
It’s an incredible opportunity to connect with global leaders in neuro-oncology, explore groundbreaking research, and contribute to important conversations shaping the future of our field.
Looking forward to insightful sessions, meaningful collaborations, and inspiration all around!
Mahalo to the organizers and community for bringing us together.”

More posts featuring Elias Ortega Chahla on OncoDaily.


