Patrick Soon-Shiong, Chairman of Chan Soon-Shiong Family Foundation, Executive Chairman at ImmunityBio, and Executive Chairman of the Los Angeles Times, shared a post on X:
“Every cancer patient undergoes a complete blood count. Within that routine report is a measurement that almost never makes its way into clinical conversations, yet it carries independent prognostic weight that often exceeds imaging, molecular markers, or tumor stage. That measurement is the absolute lymphocyte count, the readout of the circulating natural killer cells and T cells responsible for controlling malignant growth. When that number drops below 1,000 cells per microliter, the body enters a state of immune failure known as lymphopenia. In that state, the cellular machinery required to restrain cancer is no longer available.

Lymphopenia represents a critical, widely unaddressed problem. Modern oncology regularly intervenes to reverse anemia or neutropenia, because decades of investment created drugs capable of restoring those cell populations. But when the lymphocyte compartment collapses, physicians have had no approved way to rebuild it. As a result, the most important immunologic marker in cancer care has remained largely unacted upon. The decline of the body’s cancer-killing lymphocytes has been observed, documented, and then largely ignored.
The consequences of this clinical blind spot have been well documented across medical literature. Ray-Coquard and colleagues showed in a 2009 Cancer Research analysis that lymphopenia predicted inferior survival across multiple solid tumors, cutting across anatomic and histologic boundaries. In lung cancer, Abravan and co-authors demonstrated that profound treatment-related lymphopenia was associated with sharply worse overall survival, even after adjusting for traditional variables. In immunotherapy, severe lymphopenia has repeatedly been shown to predict early failure. Ménétrier-Caux et al. (2019) reported that depletion of circulating lymphocytes acts as a surrogate marker for initial resistance to immune-checkpoint inhibition.
Other specialties have reported the same biological signal. Investigators at the Mayo Clinic have shown that patients with glioblastoma have nearly double the mortality risk when their ALC drops below 750.
Breast cancer researchers at UCLA found that among many clinical and genomic variables, the most powerful predictor of future metastasis was a low baseline lymphocyte count. This link between lymphocyte depletion and mortality extends well beyond cancer. In sepsis, Drewry’s Critical Care study (2014) showed that persistent lymphopenia predicted death more reliably than commonly used severity scoring systems. In COVID-19, Li et al. (2020) demonstrated that patients with severe disease exhibit marked losses of NK cells and CD8⁺ T cells, accompanied by exhaustion phenotypes that predicted early clinical deterioration.
This recurring observation – diminished lymphocytes, worsened outcomes – is not a coincidence. It is a reflection of immune physiology. Natural killer cells and CD8⁺ T cells form the core of the body’s ability to detect and eliminate malignant or infected cells. These cells are not ancillary. They are essential for survival. Without them, tumors progress unchecked, infections worsen, and no therapeutic platform can achieve durable control.
For many years, the field pursued the wrong cytokine to rebuild this system. Interleukin-2 (IL-2) was widely believed to be the central growth factor for cytotoxic T cells. In reality, IL-2 drives expansion of regulatory T cells, a population that suppresses immune activity. The result was decades of treatments built on an incomplete understanding of cytokine biology. High-dose IL-2, approved by the FDA in 1992, was administered without recognition that it amplified suppressive T-cell populations at the expense of cancer-killing cells.
Only in the past fifteen years has the correct biology come into view. Interleukin-15 (IL-15), not IL-2, is the principal cytokine responsible for expanding NK cells and CD8⁺ T cells without simultaneously expanding regulatory T cells. In 2008, the National Cancer Institute ranked IL-15 as the most promising cytokine for therapeutic development. That insight eventually led to the emergence of IL-15–based agents that can reliably and safely expand the effector lymphocytes required for cancer control. By 2024, therapies capable of restoring NK and CD8⁺ T-cell levels without triggering compensatory immune suppression reached clinical practice with FDA’s first approval of ANKTIVA for bladder cancer, the world’s first IL-15 stimulating protein for expansion of killer cells (NK and T cells).
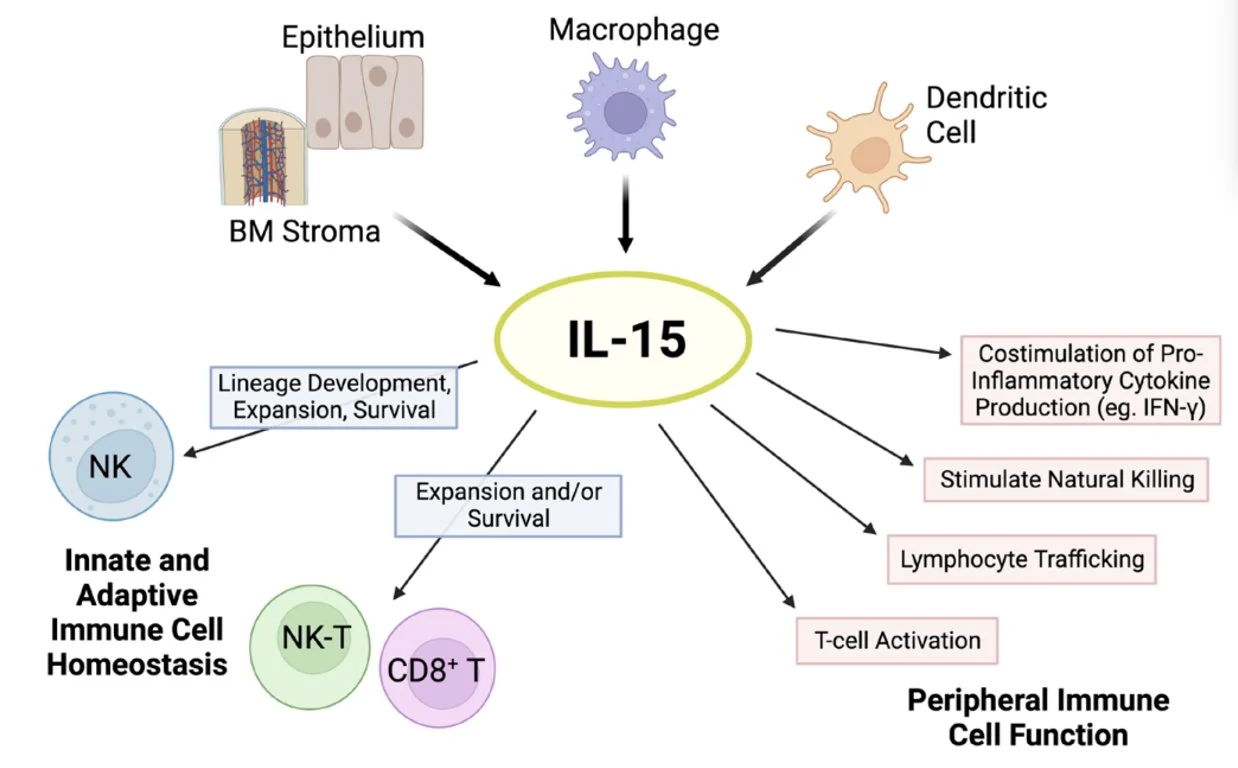
Effects of IL-15. The cytokine IL-15 is produced by multiple cell types, such as activated dendritic cells, monocytes/macrophages, epithelial cells, and bone marrow (BM) stromal cells. IL-15 is critical for natural killer (NK) cell lineage development, survival, and proliferation. This cytokine holds important modulatory effects in the expansion and survival of CD8+ T cells, including memory formation, and lymphocyte cytotoxic function. In addition, IL-15 has pleiotropic effects on peripheral innate and adaptive immunity.
Across tumor types, clinical studies have converged on the same finding: patients who regain their lymphocyte counts experience significantly longer survival than those who remain severely depleted. The survival advantage reflects biology, not confounding. An immune system that has lost its operative killer cells cannot maintain tumor control or synergies with modern therapeutics. Restoring these cells restores the possibility of long-term survival.
Recognizing lymphocytes as an organ system at risk opens a path toward a different oncology model. Chemotherapy and radiation-delivered for decades at the “maximum tolerated dose,” the highest exposure a patient can survive – are now understood to cause profound immune injury. These treatments need not be abandoned; they must be modernized. At calibrated, biologically informed doses, cytotoxic therapy can expose stressed tumor antigens while sparing the lymphocyte pool.
When paired with IL-15–based expansion of NK and T cells, this approach converts standard therapies into potent immunologic amplifiers rather than immunologic liabilities.
This shift, however, collides directly with a regulatory architecture designed for another era. The FDA has clear frameworks for approving interventions that restore red blood cells or neutrophils.
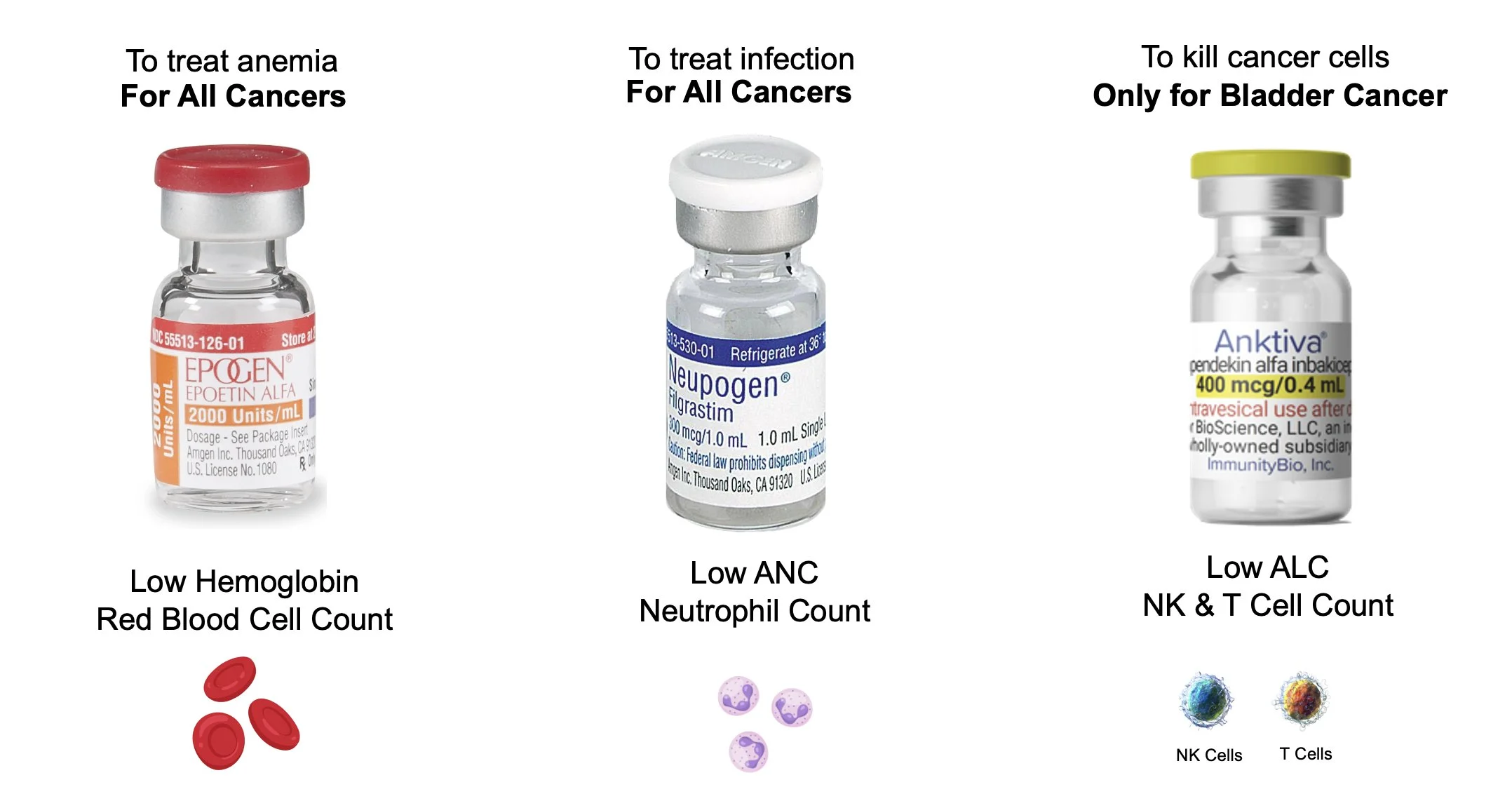
No such template exists for restoring lymphocytes, despite decades of literature demonstrating the central role of lymphopenia in cancer mortality. Regulators lack a precedent for evaluating therapies whose primary target is immune restoration rather than tumor size.
FDA’s New Plausible Mechanism Pathway: Light at the End of the Tunnel?
Published November 2025
The FDA has acknowledged the need for updated approaches. In a recent New England Journal of Medicine perspective, Commissioner Marty Makary and Dr. Vinay Prasad, Chief Medical and Scientific Officer and Director of the Center for Biologics Evaluation and Research (CBER) called for flexible evaluation pathways for therapies supported by strong mechanistic rationale, demonstrable biological plausibility, and urgent unmet medical need. The agency noted that traditional multi-year, tumor-specific randomized trials may be impractical when the underlying biological defect is universal across cancers.
Their message was explicit:
When mechanism and unmet need align, the regulatory process must adapt.
This is not a call for less evidence; it is a call for the correct evidence. When lymphocyte collapse is the physiological driver of mortality, and when a therapy directly restores that compartment, the restoration itself becomes a clinically meaningful endpoint. Accelerated approval frameworks exist to accommodate exactly this type of biologically driven therapeutic class, with confirmatory studies proceeding in parallel.
Several steps are required to bring practice, policy, and biology into alignment.
1. Treat lymphocyte counts as essential clinical data.
ALC should be tracked with the same seriousness as hemoglobin or neutrophil counts. Values below 1,000 must be recognized as high-risk and addressed proactively.
2. Build evidence registries that reflect clinical reality.
Randomized placebo-controlled trials are no longer ethical in many late-stage settings. Evidence registries—long used in cardiology and infectious disease—can track outcomes from immune-restorative therapies at scale. CMS’s Coverage with Evidence Development pathway already permits this model.
3. Update regulatory frameworks to incorporate immune restoration.
When a therapy targets a well-defined biological mechanism, has an established safety foundation, and addresses a lethal condition with no approved treatment, accelerated approval with confirmatory follow-up is the appropriate path. The tools exist. They must be used.
Oncology is entering a new phase. The field is beginning to recognize that defeating cancer is not merely about targeting malignant cells; it is about ensuring that the immune system remains intact enough to participate in that fight.
Once the lymphocyte compartment collapses, no drug can compensate.
The warning has been visible for decades, printed in plain text on every CBC panel run for every cancer patient in the country. If clinicians measure it, if regulators recognize it, and if the system supports restoring it, patient survival can change at scale.
The evidence is consistent. The risk is quantifiable. The therapeutic opportunity is now. Lymphopenia can no longer be ignored. A treatment exists. Lives are at stake. Cancer is a war against time.
This administration can act to change the course of cancer for millions of Americans and lead the world in innovation.
Here are some of the dramatic examples when patients have access to the Cancer Bioshield protocol
Multiple tumor types
Since 2015, over 26 clinical trials with over 8,000 doses of ANKTIVA across multiple cancer types with consistent results of long-duration of response.
Non-Muscle Invasive Bladder Cancer (NMIBC)
Over nine years free of non-muscle invasive bladder cancer, papillary. A clinical trial in bladder cancer together with registration trials now over ten years. Approved for a small subset of NMIBC, carcinoma in-situ (CIS) with or without papillary. Applying to the FDA to convince the reviewers that the additional trial in NMIBC papillary without CIS with the same results as the approved trial should be granted accelerated approval to enable patients who had failed all current standards of care to save their bladder.
Head & Neck Cancer, HPV Positive, Patient #1
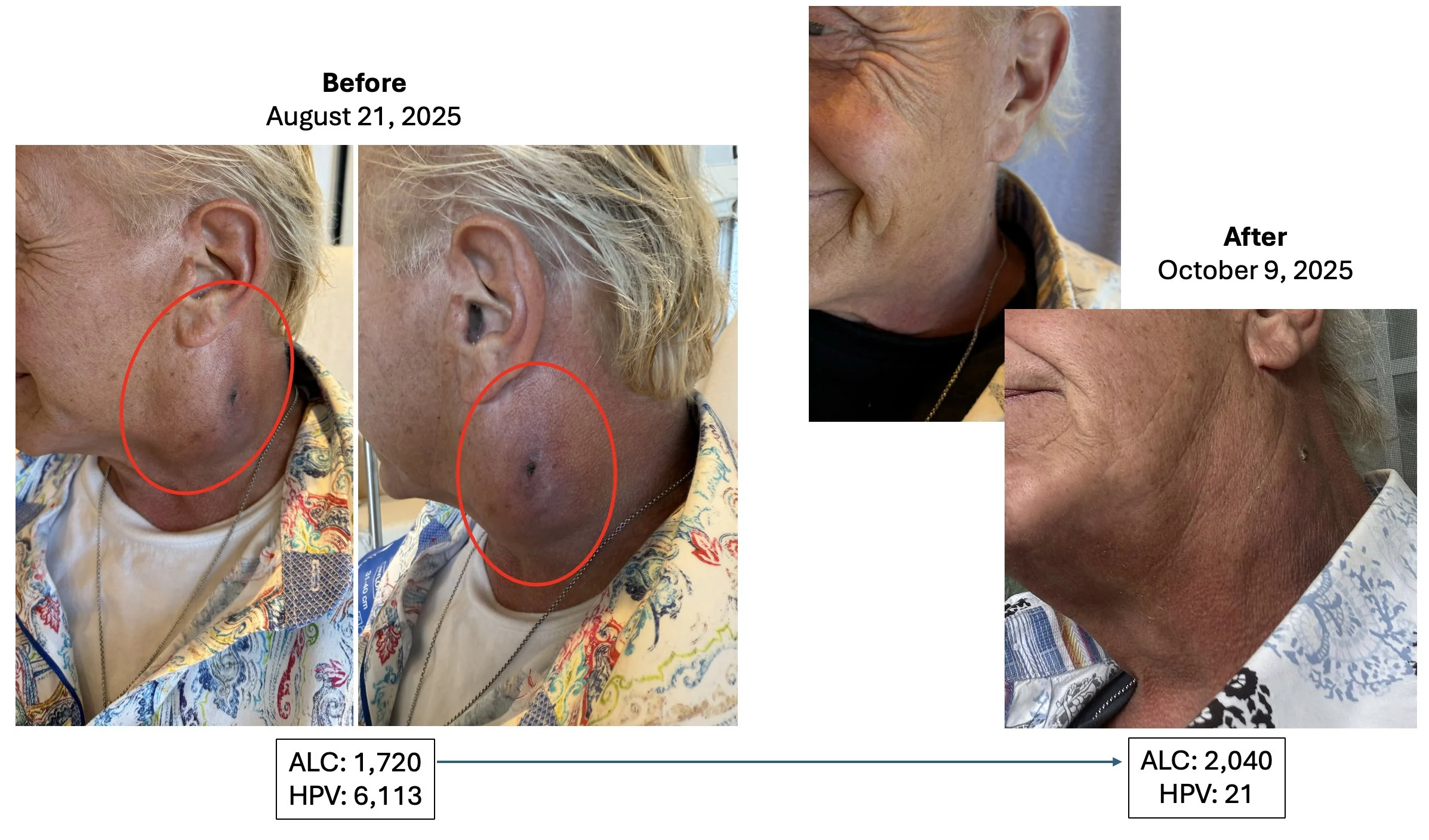
As of November 24, 2025, this patient’s CT scan confirmed complete response with no evidence of disease and an HPV level of 0 (not detectable).
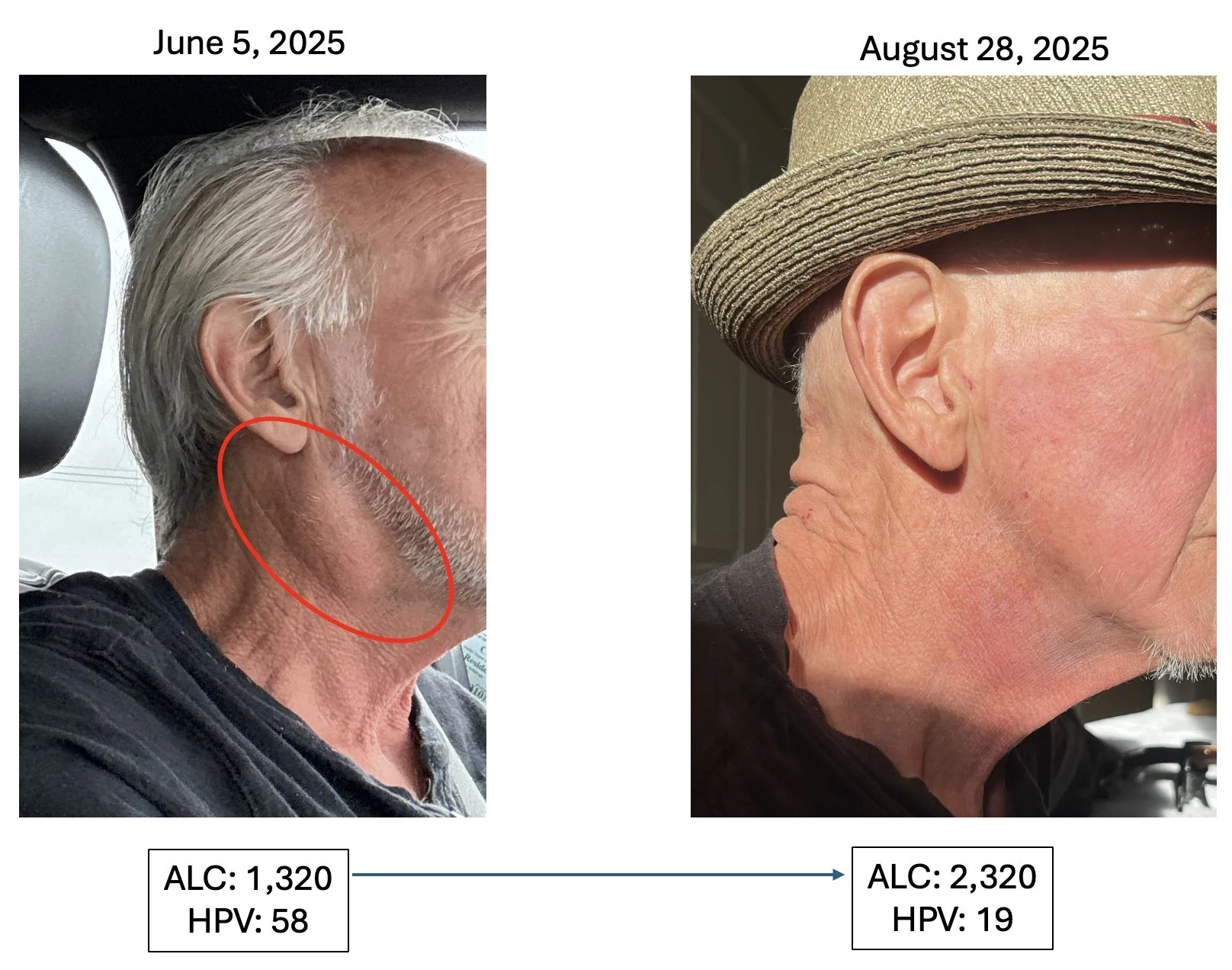
Head & Neck Cancer, HPV Positive Patient #2
Metastatic Pancreatic Cancer Patient
Stage 4 metastatic pancreatic cancer patient. Patient 6.5 years since treatment to date (as of November 24, 2025).
Recurrent Glioblastoma (GBM)
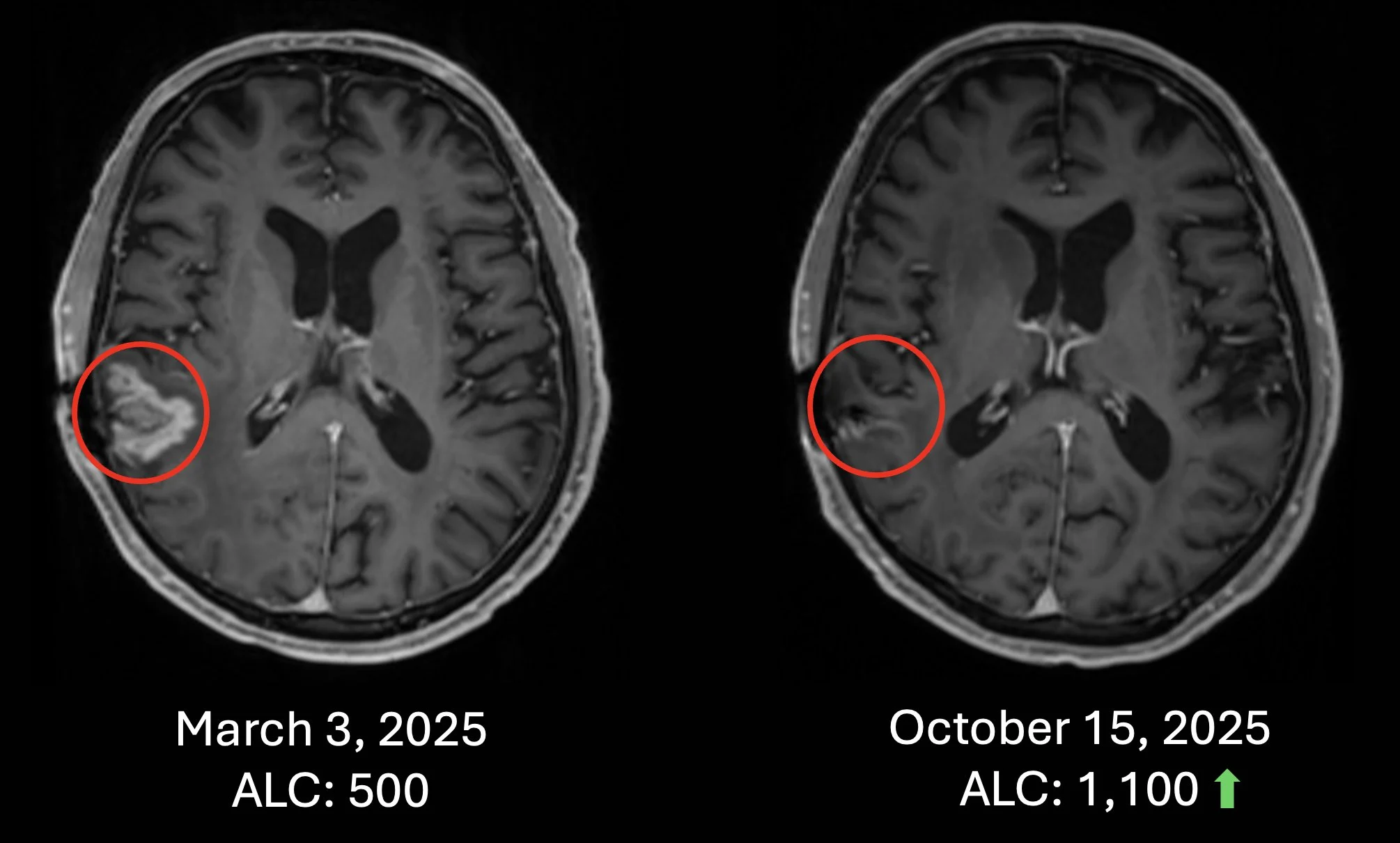
Patient #1 Recurrent Glioblastoma – Patient failed surgery, radiation and chemotherapy. Recurrence in March 2025 and received chemotherapy-free Cancer Bioshield protocol with increase of ALC and with almost complete response (October 15, 2025). Treatment ongoing to date (as of Nov 24, 2025).
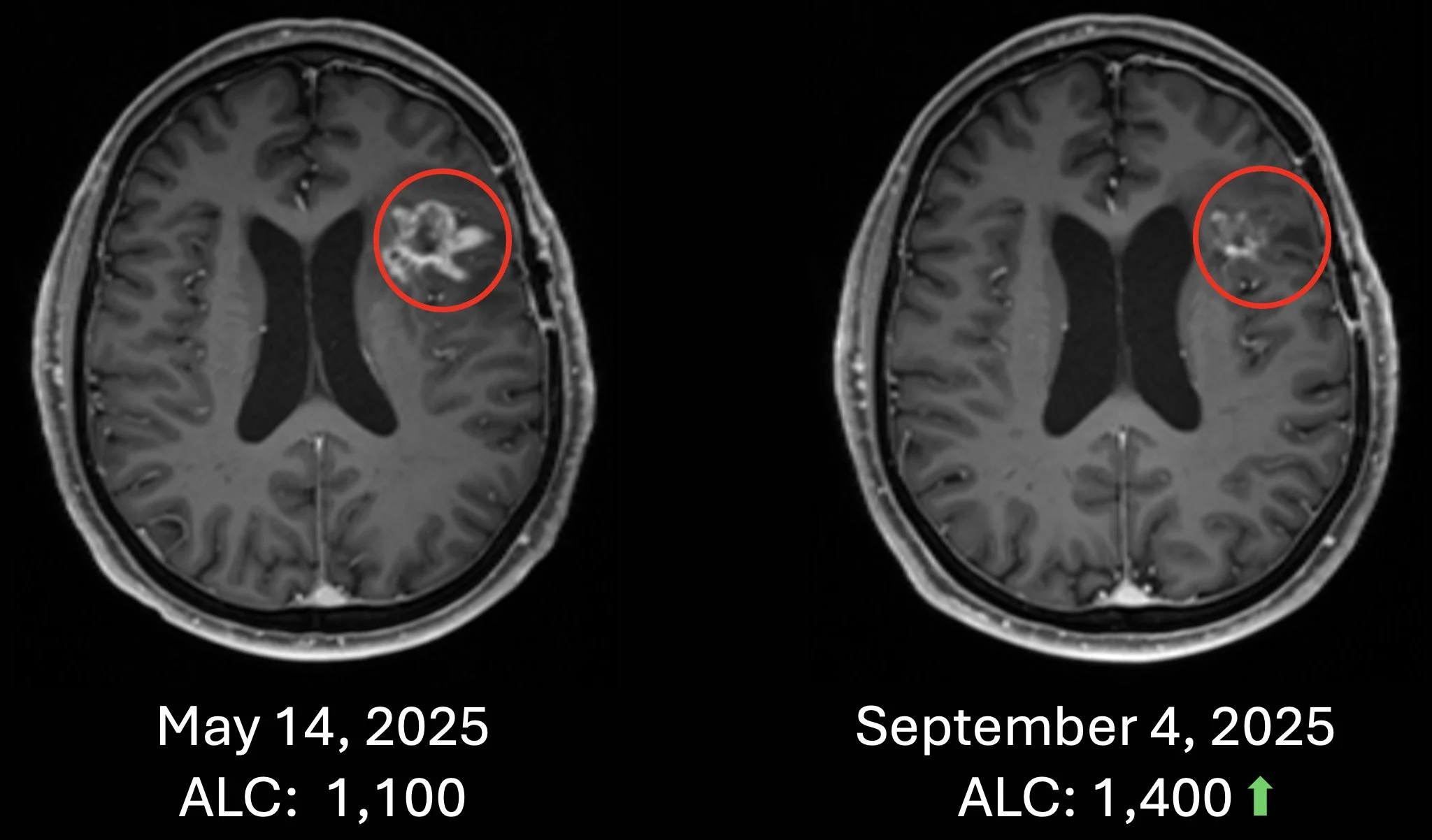
Patient #2 Recurrent Glioblastoma – Patient failed surgery, radiation and chemotherapy. Recurrence in May 2025 and received chemotherapy-free Cancer Bioshield protocol with increase of ALC with significant response (Sept 2025). Treatment ongoing to date (as of Nov 24, 2025).
Recurrent Choroid Plexus Brain Tumor
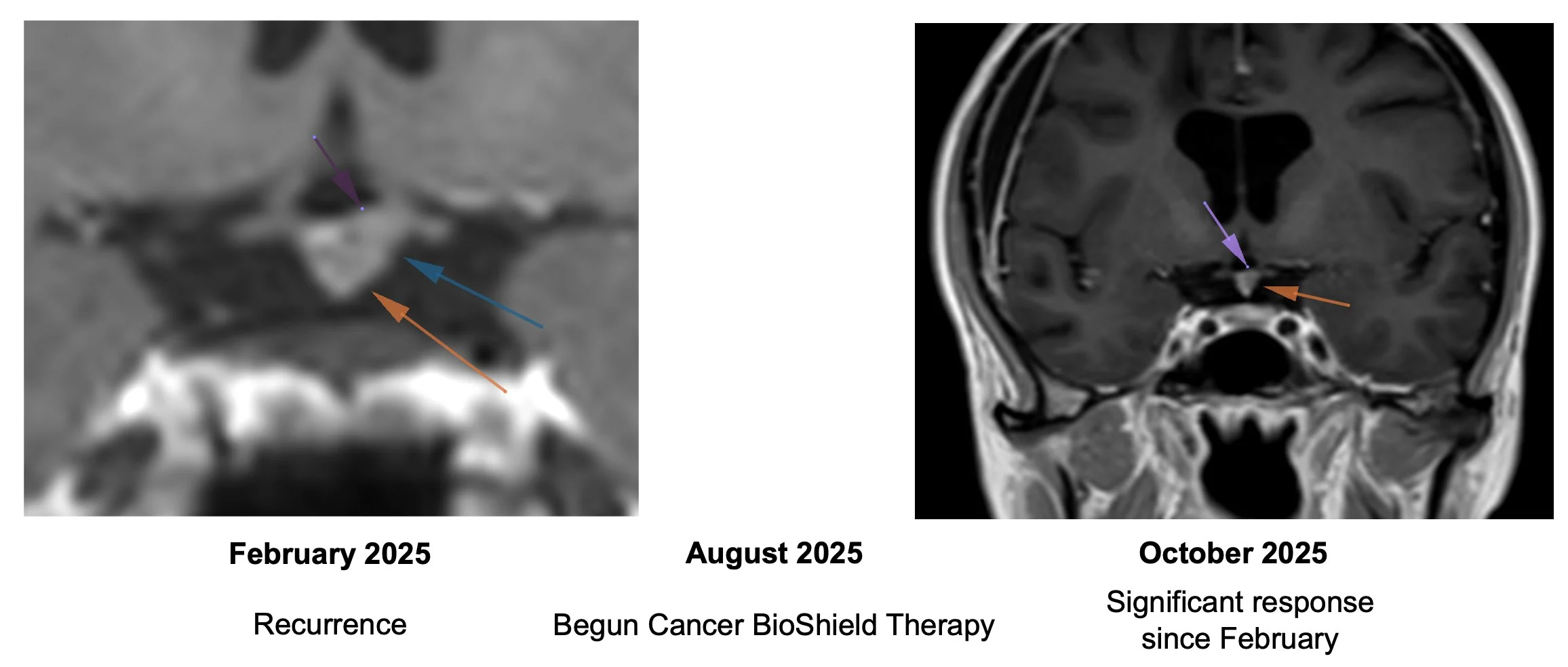
Began Cancer Bioshield therapy on August 2025 with significant response by October 2025.
Recurrent Prostate Cancer
Waldenstrom’s Lymphoma
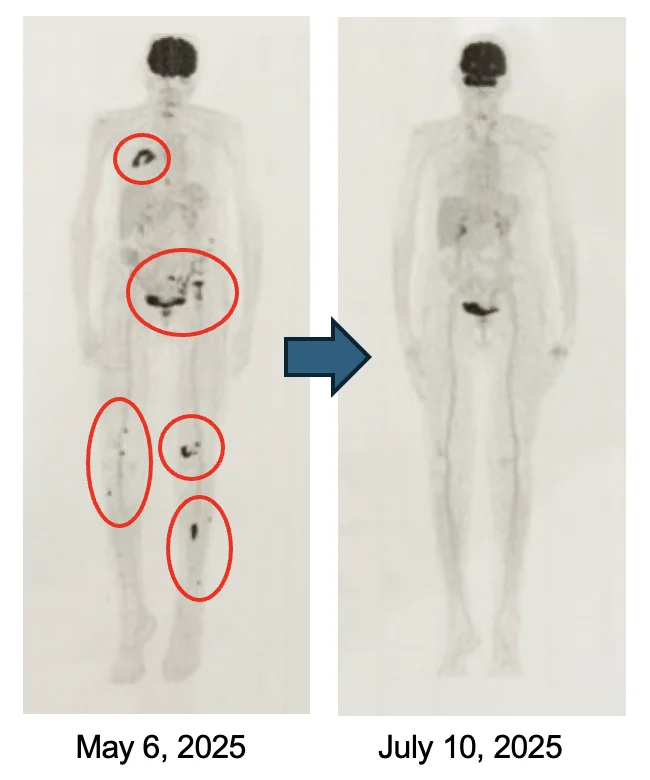
Complete response with Cancer Bioshield protocol.
Clinical Trials at CSSIFM
- Glioblastoma
- Head & Neck Cancer
- Lung Cancer
- Non-Hodgkin’s Lymphoma
- Waldenstrom’s Lymphoma
- Prostate Cancer
- Expanded Access Lymphopenia
- Long-COVID Trial
For more information, please visit CSSIFM.org or email at info@cssifm.org.”
More posts featuring Patrick Soon-Shiong on OncoDaily.


