
New Paper Alert!- PARP Inhibitor Rucaparib Shows Promise in Metastatic Hormone-Sensitive Prostate Cancer with HRR Mutations
PARP Inhibitor Rucaparib Shows Promise in Metastatic Hormone-Sensitive Prostate Cancer with HRR Mutations
Authors: Mark C Markowski, Cora N Sternberg, Hao Wang, Tingchang Wang, Laura Linville, Catherine H Marshall, Rana Sullivan, Serina King, Tamara L Lotan, Emmanuel S Antonarakis
Published in The Oncologist on June 17, 2024
Introduction:
The TRIUMPH study explored the efficacy of rucaparib, a PARP inhibitor, in patients with metastatic hormone-sensitive prostate cancer (mHSPC) harboring germline homologous recombination repair (HRR) gene mutations.
Previous research has shown PARP inhibitors to be effective in metastatic castration-resistant prostate cancer (mCRPC) when combined with androgen deprivation therapy (ADT). This phase II trial assessed whether rucaparib could be effective without concurrent ADT in mHSPC patients with HRR mutations.
Design and Methods:
This multi-center, single-arm phase II trial enrolled 12 patients with asymptomatic mHSPC and pathogenic germline HRR mutations.
Participants received rucaparib 600 mg orally twice daily without ADT. The primary endpoint was a confirmed PSA50 response rate (≥50% decline in PSA from baseline, confirmed after 4 weeks).
Secondary endpoints included objective response rate (ORR), radiographic progression-free survival (rPFS), PSA progression-free survival (PSA-PFS), and safety assessment.
Key Results:
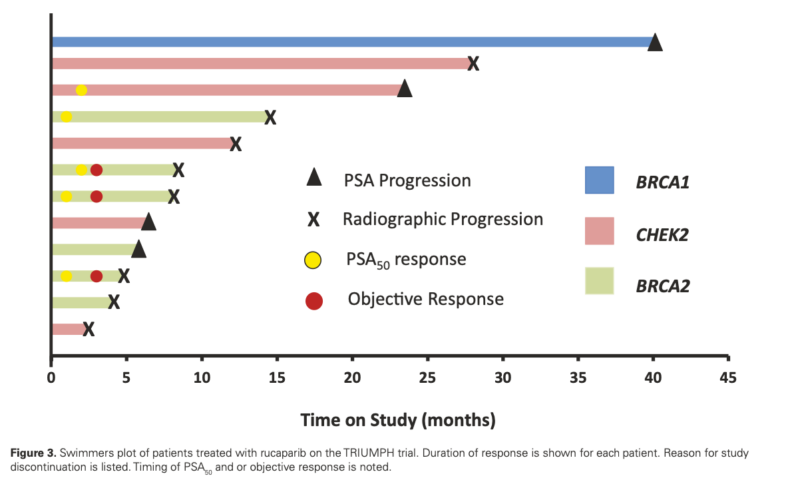
- PSA50 response rate: 41.7% (5/12 patients, 95% CI: 15.2-72.3%)
- ORR in patients with measurable disease: 60% (3/5 patients, 95% CI: 14.7-94.7%)
- Median rPFS: 12.0 months (95% CI: 8.0-not reached)
- Median PSA-PFS: 11.2 months (95% CI: 3.7-not reached)
- BRCA2 mutation carriers: 4/6 (67%) achieved PSA50 response
- Most common adverse events: fatigue (58%) and transaminitis (50%), all Grade ≤2
What We Learned:
The study demonstrated that rucaparib can induce clinical responses in mHSPC patients with HRR mutations without concurrent ADT. However, the pre-specified efficacy threshold (PSA50 response rate of 75%) was unmet, leading to early trial termination.
Notably, patients with BRCA2 mutations showed a higher response rate (67%) than those with CHEK2 mutations. The study also revealed that biallelic HRR gene inactivation might be crucial for treatment response, as patients without confirmed biallelic inactivation showed poorer outcomes.
Key Highlights:
- First trial showing clinical activity of PARP inhibition in metastatic prostate cancer without ADT
- Median rPFS of 12 months, comparable to results in mCRPC studies (TRITON2 and TRITON3)
- Higher response rates in BRCA2 mutation carriers compared to CHEK2 mutation carriers
- Importance of biallelic HRR gene inactivation for treatment response
- Generally well-tolerated safety profile with mostly Grade ≤2 adverse events.
Key Takeaway Messages:
- Rucaparib shows potential as a non-hormonal therapy option for mHSPC patients with HRR mutations, particularly those with BRCA2 mutations.
- The study provides proof-of-principle that PARP inhibition alone can induce responses in biomarker-selected metastatic prostate cancer patients.
- Biallelic HRR gene inactivation may be a crucial factor in determining treatment response to PARP inhibitors.
- While showing promise, PARP inhibition alone is unlikely to replace ADT-based treatment paradigms for first-line mHSPC therapy.
- Further research is needed to optimize patient selection and explore combination strategies with PARP inhibitors in mHSPC.
The TRIUMPH study, despite not meeting its primary endpoint, provides valuable insights into the potential of PARP inhibitors as a hormone-sparing approach in mHSPC.
The observed clinical activity, particularly in BRCA2 mutation carriers, suggests that targeting AR may not always be necessary for response in HRR-mutated prostate cancer. However, the study’s small sample size and early termination limit the generalizability of its findings.
These results, combined with ongoing trials exploring PARP inhibitor combinations with ADT and novel AR-targeted therapies, contribute to our understanding of optimal treatment strategies for HRR-mutated prostate cancer.
While PARP inhibition alone may not supplant current first-line treatments for mHSPC, it could potentially offer an alternative approach for select patients, allowing them to defer hormonal therapies.
Future research should focus on refining patient selection criteria, particularly regarding biallelic HRR gene inactivation, and exploring combination strategies to maximize the potential of PARP inhibitors in prostate cancer treatment.
Summary by Amalya Sargsyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
