
Nitin Jain: Pirtobrutinib Venetoclax and Obinutuzumab in First-Line treatment for CLL
Nitin Jain shared on X: .
“Delighted to present results of an investigator-initiated trial of Pirtobrutinib and Venetoclax and Obinutuzumab as first-line treatment of patients with Chronic lymphocytic leukemia (CLL) at EHA2024 (European Hematology Association).

- Several trials previously combined covalent BTKi (ibr, acala, zanu) with BCL2i venetoclax +/- CD20 mAb obinutuzumab
- We report first results of non covalent BTKi pirtobrutinib, ven and obin as time limited approach in first line CLL for patients meeting iwCLL (International Workshop on Chronic Lymphocytic Leukemia) treatment indications
Here is the treatment schema NGS MRD assessed at 10-6 sensitivity in both blood and marrow at serial time points.
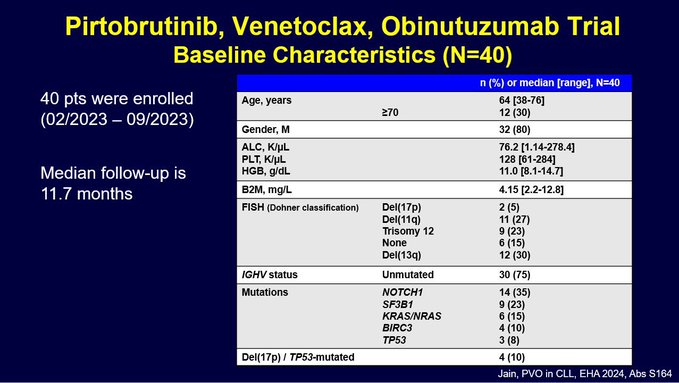
Here are the baseline characteristics.
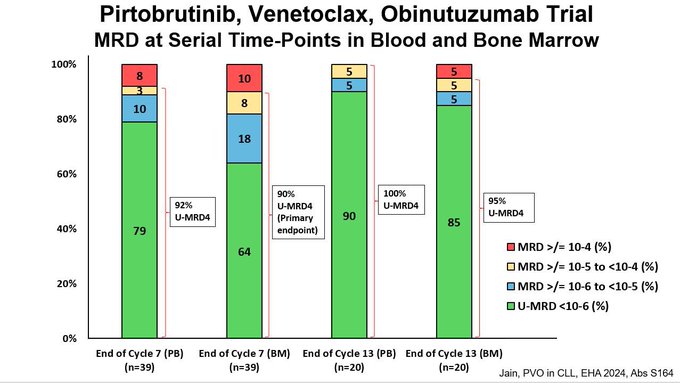
- This slide shows NGS MRD rates at end of Cycle 7 and 13 for both blood and marrow.
Green color is NGS undetectable at 10-6 sensitivity.
- Overall very high rates of U-MRD6!
Data is consistent across time points and sample site (blood and marrow).
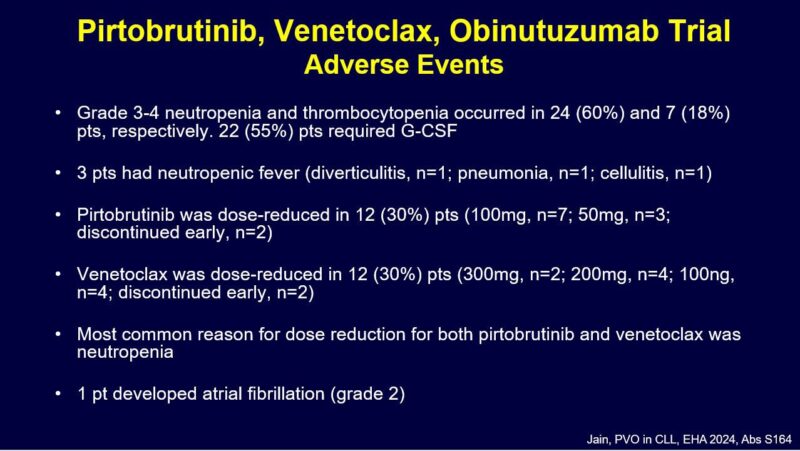
Here are key toxicities.
Here are the study conclusions.

- So how do these results compare to other doublets/triplets in first-line CLL?
- Here is a summary slide I made for comparison at about 1 year of combination treatment.
Data listed for blood and marrow at different levels of MRD as best I could gather from papers/abstracts.
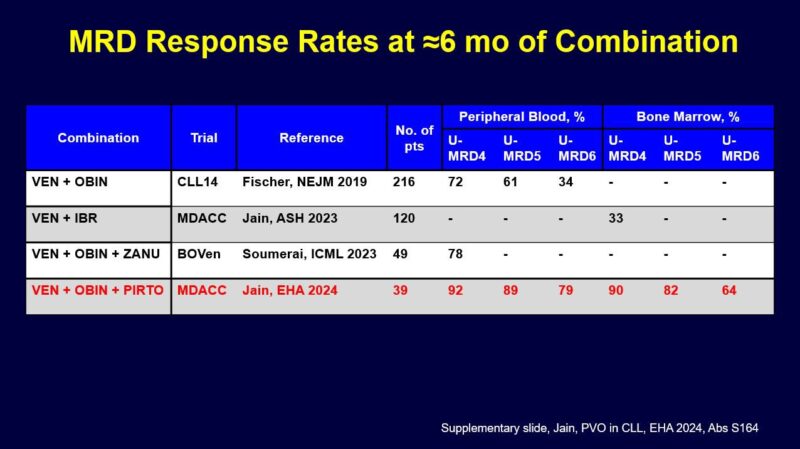
Here is another slide looking at an earlier time point of about 6 months. One caveat is that this is cross trial comparison and some trials enrolled high risk genomics patients (MDACC I+V, CLL2-GIVe) but I think these comparison slides are helpful as we think about these regimens.
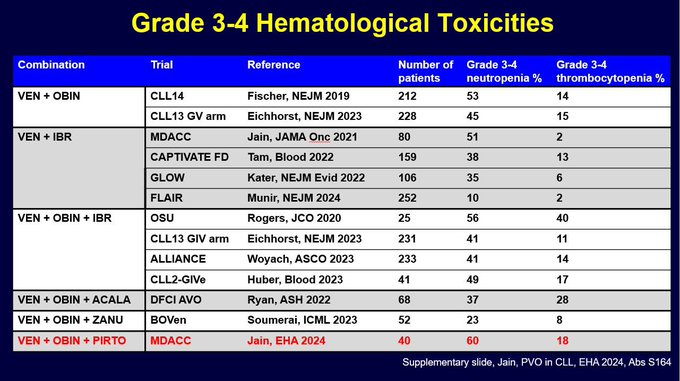
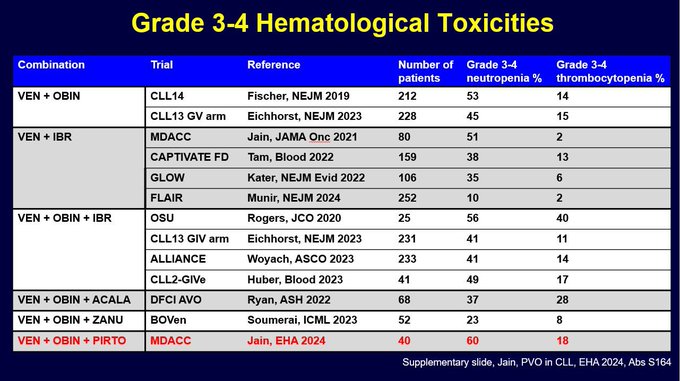
I was asked about rates of G3-4 neutropenia and thrombocytopenia. So I made this summary slide (took a while to gather this data from papers and abstract presentations). It is clear rates of G3-4 ANC are around 40-60%. And obin containing regimens have G3-4 PLT in 10-20% range.
A big Thanks to patients and families for ongoing participation in this trial. Thanks to Eli Lilly team for their excellent collaboration on this trial.”

Source: Nitin Jain/X
Dr. Nitin Jain is a Professor of Medicine at The University of Texas MD Anderson Cancer Center, Houston, USA. He leads the CAR T program for the Leukemia Department, MDACC, and received the Leukemia and Lymphoma Society (LLS) Scholar in Clinical Research and Translational Research Program awards in 2022. Specializing in acute and chronic leukemias, notably CLL and ALL, his research focuses on new drug development, reflected in his leadership of multiple investigator-initiated clinical trials.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
