Shruti Patel, Oncology Fellow at Stanford Health Care, recently tweeted:
“Tumor Board Tuesday – Mini tweetorial
A 68-year-old woman with a history of smoking:
-Presenting with shortness of breath (SOB) and weight loss.
-Past medical history includes diabetes.
-Imaging reveals a 5.4cm mass in the right upper lobe of the lung with an ipsilateral peribronchial lymph node.
-Brain MRI shows no metastases.
-Biopsy of the lung lesion indicates adenocarcinoma of lung origin.
What would YOU do next?
- Present at Tumor Board
- Await NGS/PDL1 testing
- Platinum-based doublet
- Plat-base doublet with IO
-Stage IIIA (T3N1M0) lung adenocarcinoma.
-You present the case at a tumor board meeting.
-She has a resectable disease!
-NGS reveals no actionable mutations in the tumor’s genetic profile.
-The tumor has a programmed death-ligand 1 (PD-L1) expression of 55%.
What therapy would YOU give now?
- Surgery + adjuvant tx
- Neoadjuvant/ Plat-doublet
- Neoadjuvant/Plat-doublet+Nivo
-
Neoadjuvant/Plat-doublet+durva
Mini tweetorial 1
PERIOPERATIVE IO
National Comprehensive Cancer Network (NCCN) based NSCLC guide for biomarker negative resectable Stage IIIA.
RECOMMENDATIONS
-Surgery after neoadjuvant systemic treatment.
-Nivolumab and platinum-doublet.
Mini tweetorial 2
PERIOPERATIVE IO
Platinum Doublet Options:
-Carbo or Cisplatin + paclitaxel (any histology).
-Carbo or Cis + pemetrexed (nonsquamous).
-Carbo or Cis + gemcitabine (squamous).
Mini tweetorial 3
Nivolumab (Nivo) was approved by the FDA based on:
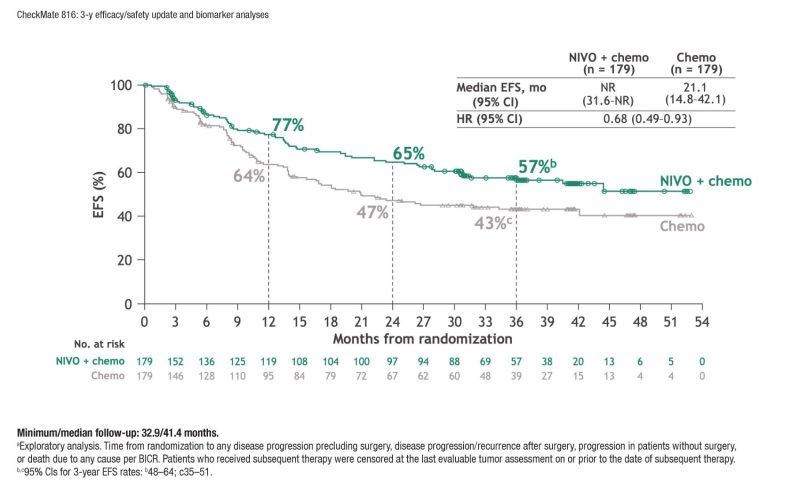
-CHECKMATE-816
-PHASE III – 3 YEAR UPDATE.
-Stage IB-IIIA (excluding EGFR & ALK mutations).
-3 rounds of chemotherapy and immunotherapy over 9 weeks, followed by surgery.
-Recurrence rate: 28% in the nivolumab + chemotherapy group versus 42% in the monotherapy chemotherapy group.
Mini tweetorial 4
Nivo approved by FDA based on:
-Improved improved pathCR rates (24% vs 2.2%).
-Decreased surgical times.
-No change in grade ≥3 adverse events.
Pending OS data?

Mini tweetorial 5
What else is being evaluated in neoadjuvant area?
–NEOSTAR
–NADIM
-Target Specific Neoadjuvant (NeoAdaura, ALK +)
Looking for neoadjuvant + adjuvant, hold your horses till tweet 12.
Mini tweetorial 6
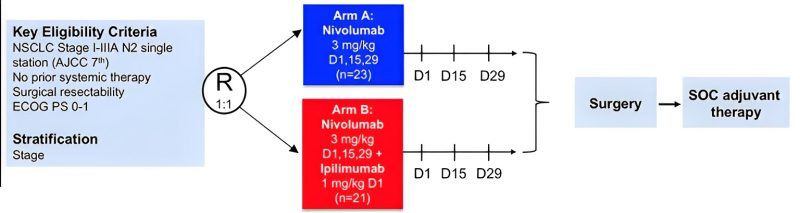
NEOSTAR
-PHASE II
-Stage I-IIIA: includes stage 1!
-Nivolumab alone vs Nivolumab + 1 dose of ipilimumab followed by surgery, and then SOC post-operative treatment.
-Adverse Events (AEs) may cause delays or cancellation of surgery.
-MPR + pCR rate was 17% in the nivolumab alone arm vs 33% in the ipilimumab/nivolumab arm.
Mini tweetorial 7
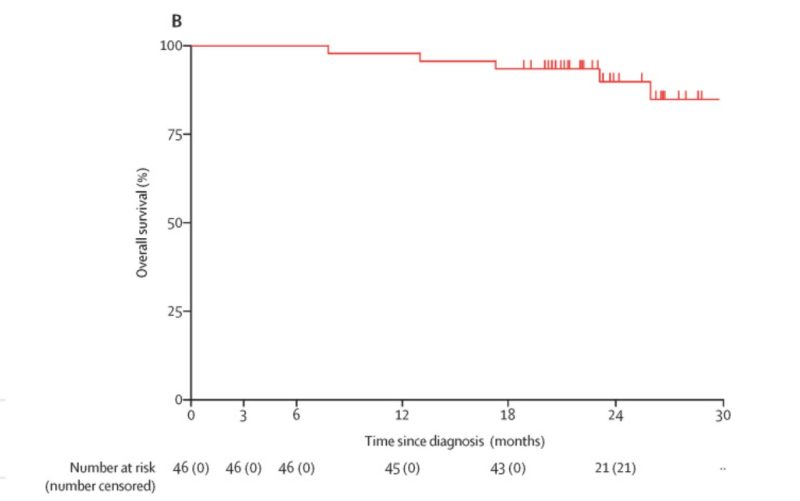
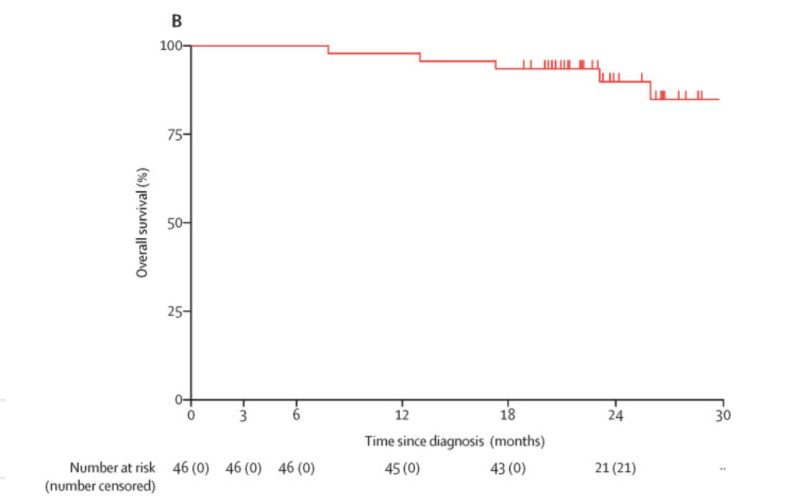
NADIM I (don’t confuse with NADIM II)
-PHASE II
-Stage IIIA (only): 51 patients
-Single Arm
-Neoadjuvant nivolumab + platinum chemotherapy vs chemotherapy (3 cycles) followed by surgery, and then patients with R0 resections receive adjuvant nivolumab for 12 months.
-Primary Endpoint: PFS at 24 months.
Back to our case!
-Patient had a discussion with her healthcare provider regarding treatment options.
-There are no contraindications for IO.
-She is considered fit for Cisplatin (geriatric assessment).
-There is concern that Cisplatin treatment may delay surgery.
-The patient decided to proceed with a treatment plan that includes Cisplatin in combination with pemetrexed and nivolumab (shared decision).
-Patient completes 3 cycles of chemotherapy with nivolumab.
-The patient developed grade I hypothyroidism and is now receiving Levothyroxine.
-A follow-up CT scan has shown a reduction in the size of the tumor.
She returns to clinic after surgery & asks: “Do I need more IV treatment, doc?”
Mini tweetorial 8
Adjuvant AFTER perioperative therapy?
Multiple studies in various stages of results:
-KEYNOTE-671
-IMpower030
-AEGEAN
-CheckMate-77T
-NADIM II

Mini tweetorial 9
KEYNOTE-671
-Phase III
-Stage II-IIIB
-4 rounds of pembrolizumab + platinum-doublet followed by surgery, and then adjuvant pembrolizumab.
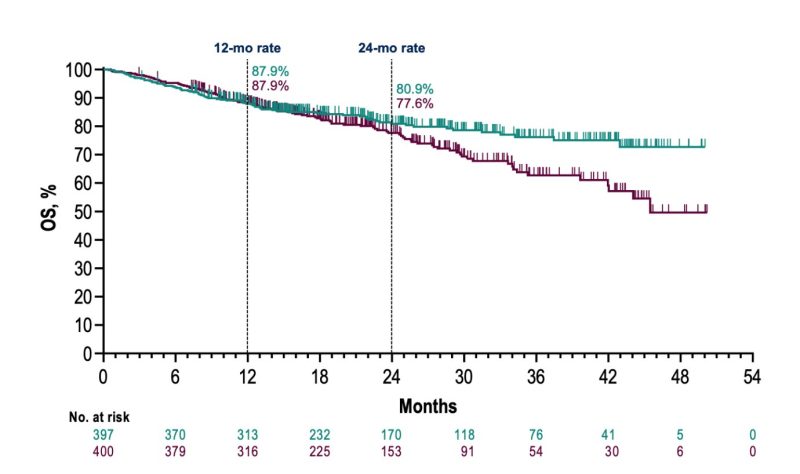
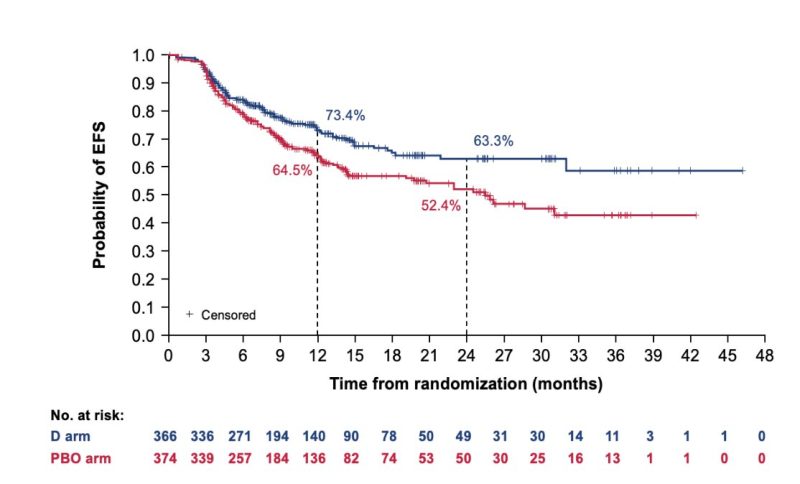
-24 month EFS rate: 62.4% in pembrolizumab + chemotherapy arm vs 40.6% in placebo arm.
-24-Month Overall Survival: 80.9% in pembrolizumab + chemotherapy arm vs 77.6% in placebo arm.
Mini tweetorial 10
-Awaiting regulatory approval.
-Improved pathCR rates (18 vs 4%).
-EFS benefit for perioperative pembrolizumab regardless of whether patients achieved pCR/mPR!
Mini tweetorial 11
-PHASE III
-Stage II-IIIB
-Treatment Arm: 4 rounds of atezolizumab + platinum-doublet followed by surgery and then adjuvant atezolizumab.
-Control Arm: 4 rounds of placebo + platinum-doublet followed by surgery and then placebo.
-Awaiting for results!
Mini tweetorial 12
AEGEAN
-PHASE III
-Stage IIA-IIIB
-Treatment Arm: 4 rounds of durvalumab + platinum-doublet followed by surgery and then adjuvant durvalumab.
-Control Arm: 4 rounds of placebo + platinum-doublet followed by surgery and then placebo.
Mini tweetorial 13
-Improved pathCR rates (17.2 vs 4.3%)
-EFS benefit with cisplatin and carboplatin, although cisplatin preferred due to better HR.
-Pending OS
Mini tweetorial 14
-PHASE III
-Stage II-IIIB
Treatment Arm: 4 rounds of nivolumab + platinum-doublet followed by surgery and then adjuvant nivolumab.
-Control Arm: 4 rounds of placebo + platinum-doublet followed by surgery and then placebo.
-Waiting for the results!

Mini tweetorial 15
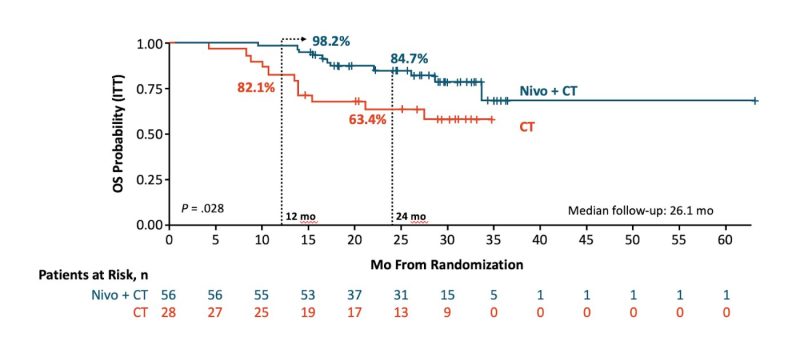
NADIM II
-STAGE IIIA and IIIB
-PHASE II
-3 rounds of nivolumab + platinum-doublet followed by surgery and then 6 months of adjuvant nivolumab.
-3 rounds of platinum-doublet followed by surgery and then by observation.
-OS – secondary endpoint.
Confused yet?
Remaining challenges!
-Duration of adjuvant IO: 6 vs 12 months
-Uniform staging.
-Does the Patients with pCR need adjuvant IO?
-Pending OS for many studies.

Caution about the following!
-NO neoadjuvant or adjuvant IO for ALK, EGFR, RET + NSCLC.
-Mandatory biomarker testing at diagnosis.
-Fertility preservation (when needed).

Neoadjuvant and Adjuvant IO are here to stay:
-Biomarker testing.
-Multi-D is essential – call your friendly surgeon.
-Geriatric assessment.
-Pending OS results.

Pros and cons!
PROS
-High mPR & pCR.
-Prolonged DFS.
-Objective eval of response.
-Increase chances of getting systemic tx.
**unclear if MPR/pCR is surrogate of OS/cure**
CONS
-AE/irAEs.
-Risk of selecting the wrong patients based on (biomarkers).
-tx discontinue.
-pending OS data.
Case outcome
-The postoperative course was unremarkable.
-Pathologic complete response.
Back to our patient’s important question, is there any adjuvant treatment?!
– It is recommended not to have adjuvant immunotherapy (IO) and instead undergo surveillance with imaging.”
Source: Shruti Patel/Twitter


