Vivek Subbiah: US FDA granted accelerated approval to tarlatamab-dlle
Vivek Subbiah shared on X:
Today the US FDA granted accelerated approval to tarlatamab-dlle (Imdelltra, Amgen, Inc.) for extensive stage small cell lung cancer (ES-SCLC) with disease progression on or after platinum-based chemotherapy.
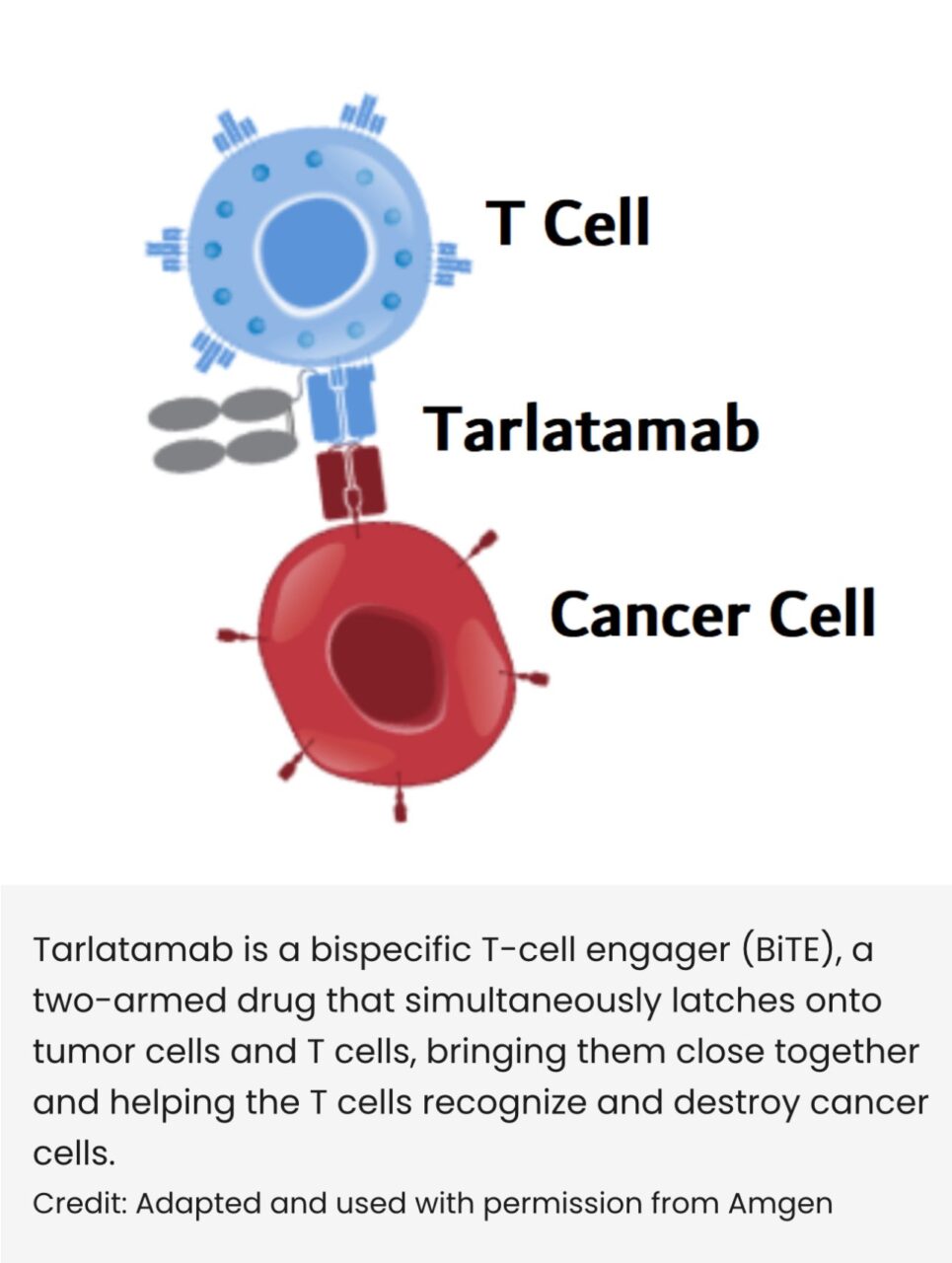
Tarlatamab = bispecific T-cell engager (BiTE), a two-armed drug that simultaneously latches onto tumor cells and T cells, bringing them close together and helping the T cells recognize and destroy cancer cells.”

Source: Vivek Subbiah/X
Vivek Subbiah is the Chief of Early-Phase Drug Development at the Sarah Cannon Research Institute (USA). He is the former Executive Director of Oncology Research at the MD Anderson Cancer Network and a former Associate Professor in the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center.
Dr. Vivek Subbiah has served as the principal investigator in over 100 phase I/II trials and co-investigator in over 200 clinical trials and is known for his leadership in several first-in-human and practice-changing studies that directly led to approvals from the FDA, European Medicines Agency, and other agencies across the world. He is an expert in tumor agnostic precision oncology and leads the BRAF and RET tissue agnostic studies to FDA approval.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
