
Innovative stem cell model provides insight into childhood cancer origins
Researchers from the University of Sheffield and St. Anna Children’s Cancer Research Institute have created a model designed to investigate the origins of neuroblastoma, a cancer primarily affecting infants and young children. The findings offer hope for the creation of tailored treatments which treat aggressive neuroblastomas and minimize the adverse effects experienced by patients from existing therapies.
Neuroblastoma is the most common solid childhood tumor occurring outside the brain, affecting the lives of approximately 600 children in the European Union and the United Kingdom. Until now, studying genetic changes and their role in neuroblastoma initiation has been challenging due to the lack of suitable laboratory methods. A new model developed by researchers at the University of Sheffield, working closely with colleagues from the St. Anna Children’s Cancer Research Institute in Vienna, replicates the emergence of early neuroblastoma cancer-like cells giving an insight into the genetic pathway of the disease.
The research, published in Nature Communications, sheds light on the intricate genetic pathways which initiate neuroblastoma. An international team, led by researchers from the University of Sheffield and St. Anna Children’s Cancer Research Institute in Vienna, found that specific mutations in chromosomes 17 and 1, combined with overactivation of the MYCN gene, play a pivotal role in the development of aggressive neuroblastoma tumors.
Childhood cancer is often diagnosed and detected late in the process, leaving researchers with very little idea of the conditions that led to tumor initiation, which occurs very early during fetal development. In order to understand tumor initiation, models which truthfully recreate the conditions that lead to the appearance of a tumor are needed.
The formation of neuroblastoma usually starts in the womb when a group of normal embryonic cells called ‘trunk neural crest (NC)’ become mutated and cancerous. In an interdisciplinary effort spearheaded by stem cell expert Dr. Ingrid Saldana (in Sheffield) and computational biologist Dr. Luis Montano (in Vienna), the new study found a way in which to use human stem cells to grow trunk NC cells in a petri dish. These cells carried genetic changes often seen in aggressive neuroblastoma tumors. Using genomics analysis and advanced imaging techniques, the researchers found that the altered cells started behaving like cancer cells and looked very similar to the neuroblastoma cells found in sick children.
The findings offer empowering insights and tools for the creation of tailored treatments that specifically target cancer while minimizing the adverse effects experienced by patients from existing therapies.
Dr. Anestis Tsakiridis, from the University of Sheffield’s School of Biosciences and one of the lead authors of the study, said: “Our stem cell-based model mimics the early stages of neuroblastoma formation, providing invaluable insights into the genetic drivers of this devastating childhood cancer. By recreating the conditions that lead to tumor initiation, we can now explore potential treatment avenues with unprecedented precision.”
Dr. Florian Halbritter, from St. Anna Children’s Cancer Research Institute and second lead author of the study, adds: “This was an impressive team effort, breaching geographic and disciplinary boundaries to enable new discoveries in childhood cancer research.”
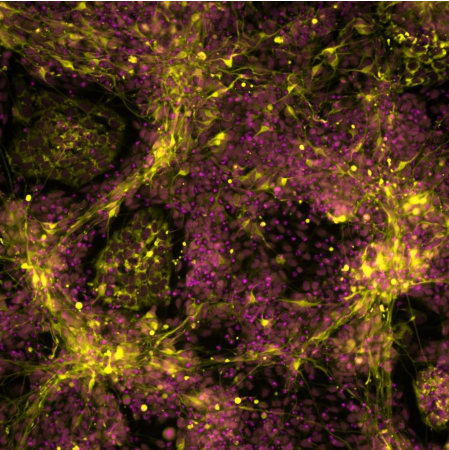
Fluorescently labelled mutant stem cells differentiated toward sympathetic neurons. Image recorded by Dr. Ingrid
Saldana.
Authors: Ingrid Saldana-Guerrero, Luis Montano-Gutierrez, Katy Boswell, Christoph Hafemeister, Evon Poon, Lisa Shaw, Dylan Stavish, Rebecca Lea, Sara Wernig-Zorc, Eva Bozsaky, Irfete Fetahu, Peter Zoescher, Ulrike Pötschger, Marie Bernkopf, Andrea Wenninger-Weinzierl, Caterina Sturtzel, Celine Souilhol, Sophia Tarelli, Mohamed Shoeb, Polyxeni Bozatzi, Magdalena Rados, Maria Guarini, Michelle Buri, Wolfgang Weninger, Eva Putz, Miller Huang, Ruth Ladenstein, Peter Andrews, Ivana Barbaric, George Cresswell, Helen Bryant, Martin Distel, Louis Chesler, Sabine Taschner-Mandl, Matthias Farlik, Anestis Tsakiridis, and Florian Halbritter.
About St. Anna Children’s Cancer Research Institute, CCRI
St. Anna CCRI is an internationally renowned multidisciplinary research institution with the aim to develop and optimize diagnostic, prognostic, and therapeutic strategies for the treatment of children and adolescents with cancer. To achieve this goal, it combines basic research with translational and clinical research, and focuses on the specific characteristics of childhood tumor diseases in order to provide young patients with the best possible and most innovative therapies. Dedicated research groups in the fields of tumor genomics and epigenomics, immunology, molecular biology, cell biology, bioinformatics and clinical research are working together to harmonize scientific findings with the clinical needs of physicians to ultimately improve the wellbeing of our patients.
About Florian Halbritter
Florian Halbritter, PhD, studied Cognitive Science at the University of Osnabrück and completed his PhD in Stem Cell Bioinformatics under the supervision of Simon Tomlinson and Ian Chambers at the MRC Centre for Regenerative Medicine at the University of Edinburgh. After graduating, he joined Christoph Bock’s lab at the CeMM Research Center for Molecular Medicine. Since 2018, he has been a Principal Investigator at St. Anna Children’s Cancer Research Institute. As a computational biologist, Florian Halbritter investigates the epigenome of stem cells, immune cells and cancer using functional genomics technologies. The aim of his research group is to better understand the earliest steps in the development of tumors and thus to pave the way for new diagnostic and therapeutic approaches.
About the University of Sheffield
The University of Sheffield, a member of the Russell Group, hosts a student population of over 30,000 from 150 countries, guided by a faculty of over 1,500 academics. Its research-driven approach informs an education focused on addressing global challenges. The university cooperates with industry in many ways.
About Anestis Tsakiridis
After completing a BSc in Biochemistry in the University of Edinburgh, Anestis Tsakiridis did a PhD in Lesley Forrester’s group in the Medical School of the same university. He then carried out his postdoctoral work, first in Josh Brickman’s lab in the Institute for Stem Cell Research and then with Val Wilson in the MRC Centre for Regenerative Medicine, both in Edinburgh. In 2016, he started his own group as a Vice Chancellor’s Fellow in the Centre for Stem Cell Biology in the School of Biosciences, University of Sheffield becoming a lecturer in 2020.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
