
David Sher Shared Results of DART Study
Amir Safavi, Head and Neck Radiation Oncologist of the Department of Radiation Oncology at the University of Toronto, shared a post by David Sher, Vice Chair of Clinical Operations and Quality, Medical Director of Radiation Oncology, Chief of Head and Neck Radiation Oncology Service at UTSW Medical Center, on X, adding:
”Phenomenally insightful and practical thread as always from David Sher on the DART study in The Lancet Oncology from Daniel Ma and Mayo Clinic Radiation Oncology team.”
Quoting David Sher’s post:
”It was such a pleasure to read the results of this juicy and terrific randomized trial of postop RT dose de-escalation for HPV+ OPSCC from Daniel Ma and colleagues at Mayo Clinic Radiation Oncology.
The field has been waiting for the formal results, and this paper does not disappoint.’
In brief, patients with resected, margin-negative HPV+ OPSCC were randomized 2:1 between 30-36 Gy BID + 2 cycles of docetaxel versus 60 Gy +/- cisplatin. Patients were stratified by smoking history and ENE. Patients without ENE were in Cohort A; those with ENE, Cohort B.
The de-escalated dose was 1.5 Gy BID, with 1.8 Gy BID delivered to stations with ENE in Cohort B. The standard dose was 60/54 Gy in 30 fractions, with Cohort B patients receiving weekly cisplatin.
The study was powered for chronic, treatment-associated grade 3 toxicity, defined as a g3+ toxicity experienced at least 3 months after radiotherapy.
It is described as a phase III trial, likely because the comparator arm was the standard-of-care, though it is an open question whether such an endpoint should be used as for a defining “phase III” study, since it was not powered for disease control.
It is important to focus on the patients included in the study, specifically clear majority never-smoking (72%!), tonsil (~60%), and in particular, 74% of DART patients had no more than a single node involved. For inclusion in the study, the patients needed to have at least one intermediate-risk pathologic factor (including 2+ nodes), so the majority of these patients presumably had PNI or LVI, since there weren’t many T3’s.
Most patients were in Cohort B (115 vs 79), including an odd duck of a pN0 patient that was in Cohort B that was supposed to have ENE.
The study was a clear success in terms of toxicity. There were statistically fewer grade 3 toxicities in the DART group, although the numbers were SMALL (kudos to the Mayo H&N team for such favorable outcomes for all): 3% (4 of 125) versus 11% (7 of 62); most of these were gastrostomy dependence, but there were some odd ones in the standard arm, such as grade 3 dry mouth and grade 3 fatigue.
The figure tells the story, both with respect to grade 3 toxicity as well as grade 2. It’s important for us to start focusing on lower-grade toxicities as well, since these side effects are the ones that stay with patients over the years.
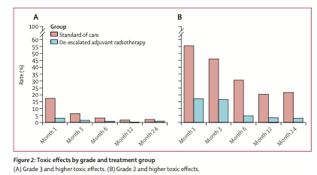
The story gets interesting with disease control, though, as PFS favored the standard arm (2 years, 96.6% vs 88.2%, p=0.02). The explanations of this difference are critical. The standard/Cohort A patients did equally well, with 100% (yes 100%) locoregional control in the 53 DART patients. However, PFS in Cohort B was 97.4% vs. 81.1% (p=0.0049), due a bit to locoregional control (100% vs. 92.9%, p=0.066) but really because of distant metastasis (97.4% vs. 90.0%, p=0.077).
The authors drilled into this group further, focusing on pN2 patients (n=25 in total), and there’s the rub. Two-year LRC with DART was only 77% and DMFS was only 60.6%, both numerically far less than the 100% in the standard group.
The authors suggest that maybe weekly cisplatin was doing something systemically that docetaxel x 2 doesn’t, but I find that unlikely. There’s never been a meaningful signal in HNSCC that concurrent chemo improves distant disease control, including the classic postop cisplatin studies. Numbers are just too tiny (16 DART, 5 SOC!) to draw meaningful conclusions there.
So what do we make of these results? First, in patients with pN2 disease, and likely pN1 with ENE, such dramatic de-escalation is not ready for prime-time. Failure rates are too high, and I don’t see a compelling reason to deviate from the standard-of-care. For the rest, clearly this DART paradigm was an absolutely HUGE win in toxicity, and the control rates were impressive. The most pressing question for me is how many of these patients really need any radiotherapy.
The primary site avoidance studies included patients with PNI/LVI without significant LF risks (admittedly with dose falloff into the oropharynx), and we would typically not consider RT just for single-node neck disease (depending some on size). So any amount of de-escalation would be expected to result in excellent outcomes because 0 Gy may be sufficient.
But if we put observation as an option aside, between the two Mayo studies we have 53 (this study) + 36 (original study) = 89 patients with intermediate-risk disease treated with 30 Gy BID + 2 cycles of docetaxel from a single institution, with world-class surgeons, pathologists and radiation oncologists to deliver the care. Is that enough for this regimen to become an accepted standard-of-care? Seems like a winning paradigm for these lower-risk patients, but ultimately we’re talking pretty tiny numbers plus these other caveats. I would love to see supportive data from other institutions, and ultimately, far more patients.
Bottom-line: amazing, amazing work from the Mayo head and neck team. Truly incredible they were able to pull this study off, and they have generated some important data for the field.”
Title: De-escalated adjuvant radiotherapy versus standard adjuvant treatment for human papillomavirus-associated oropharyngeal squamous cell carcinoma (MC1675): a phase 3, open-label, randomised controlled trial
Authors: Daniel Ma, Katharine Price, Prof Eric Moore, Prof Samir Patel, Prof Michael Hinni, David Routman, Briant Fruth, Nathan Foster, Prof Kathryn Van Abel, Linda Yin, Michelle Neben-Wittich, Yolanda Garces, Lisa McGee, Scott Lester, Jean-Claude Rwigema, Adam Holtzman, Prof Daniel Price, Prof Jeffrey Janus, Prof Jan Kasperbauer, Ashish Chintakuntlawar, Prof Joaquin Garcia, Prof Robert Foote
Read the Full Article.
More posts featuring Amir Safavi.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
