
New IO Paper Alert: Neutrophil-to-Lymphocyte Ratio as a Predictive Biomarker for First-Line Immunotherapy Outcomes in HCC.
Hepatocellular carcinoma (HCC) is one of the most common and deadly cancers worldwide, often diagnosed at advanced stages when systemic therapies are the mainstay of treatment. Despite progress with immune checkpoint inhibitors, such as atezolizumab plus bevacizumab and durvalumab plus tremelimumab, many patients still experience limited benefit, and no validated biomarkers exist to guide treatment selection. The study “Neutrophil-to-Lymphocyte Ratio (NLR) as a Predictive Biomarker in Advanced HCC Treated with First-Line Immunotherapy” investigated whether baseline NLR, a simple measure of systemic inflammation, could serve as a cost-effective predictor of treatment outcomes.
Title: Neutrophil-to-Lymphocyte Ratio (NLR) as a Predictive Biomarker in Advanced Hepatocellular Carcinoma Treated with First-Line Immunotherapy.
Authors: Tiago Felismino, Luanna Martins, Matheus Barroso, Daniela Carvalho, Angelo Brito, Claudia Maccali
Published in Journal of Gastrointestinal Cancer
Background
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and a leading cause of cancer-related mortality worldwide. While systemic therapy with immune checkpoint inhibitors (ICIs), either alone or in combination with targeted agents, has reshaped treatment for advanced HCC, response rates remain modest, and no validated predictive biomarkers are available.
The neutrophil-to-lymphocyte ratio (NLR), derived from routine blood counts, reflects systemic inflammation and immune competence. Elevated NLR has been linked to poor survival in multiple cancers, but most evidence comes from Asian, hepatitis B virus (HBV)-driven cohorts. The present study provides real-world data from a Latin American population, predominantly with non-viral HCC, to assess the role of baseline NLR as a predictive biomarker for immunotherapy outcomes
Methods
This was a retrospective, single-center study conducted at the A.C. Camargo Cancer Center in São Paulo, Brazil. Consecutive adult patients with radiologically or histologically confirmed advanced HCC who initiated first-line immunotherapy between July 2020 and March 2025 were included. Treatments consisted of atezolizumab plus bevacizumab or durvalumab plus tremelimumab, both standard first-line regimens.
Baseline NLR was calculated using pretreatment complete blood counts, excluding patients with infections within 30 days to avoid confounding. Maximally selected rank statistics identified the optimal NLR threshold for progression-free survival (PFS), which was set at 4 for clinical applicability.
Outcomes measured included progression-free survival (PFS), overall survival (OS), and disease control rate (DCR). PFS and OS were estimated by Kaplan–Meier method and compared by log-rank test. Univariable and multivariable Cox proportional hazards models assessed prognostic factors. Logistic regression evaluated the association between NLR and DCR
Study Design and Patient Characteristics
- Fifty-eight patients were analyzed, with a median age of 69 years (range 42–90); 43.1% were ≥70 years. The majority were male (82.8%). ECOG performance status was 0 in 62.1% and 1 in 37.9%. Most patients had well-preserved liver function: Child–Pugh A in 82.7%.
- Non-viral etiology predominated (72.4%), while only 27.6% had viral hepatitis-related HCC. The disease was advanced in most cases, with Barcelona Clinic Liver Cancer (BCLC) stage C in 75.9% and vascular invasion present in 44.8%.
- Regarding treatment, 48 patients (82.8%) received atezolizumab–bevacizumab and 10 (17.2%) received durvalumab–tremelimumab.
- The optimal cut-off for baseline NLR was 3.39, rounded to 4. At baseline, 28.8% of patients (15/58) had NLR ≥ 4.
Results
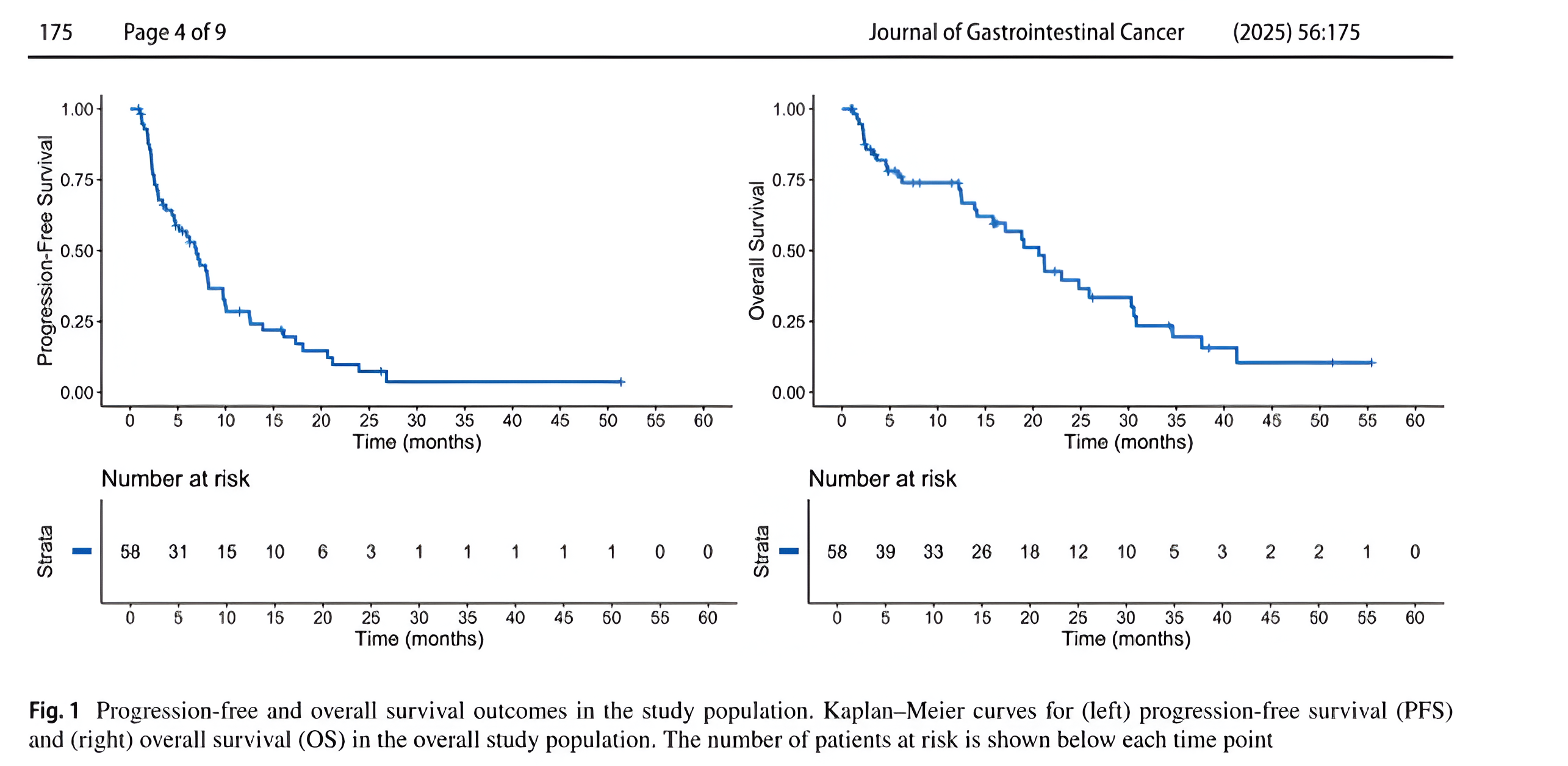
After a median follow-up of 34.3 months, 48 PFS events and 35 OS events were recorded.
Overall outcomes:
- Median PFS: 6.9 months (95% CI, 4.6–9.7).
- 12-month PFS rate: 28.5% (95% CI, 18.5–44.0).
- Median OS: 20.6 months (95% CI, 14.1–30.3).
- 12-month OS rate: 73.9% (95% CI, 63.0–86.7).
- Radiological responses (n=53 evaluable):
- Complete response (CR): 13.2%
- Partial response (PR): 24.5%
- Stable disease (SD): 47.2%
- Progressive disease (PD): 15.1%
- Disease control rate (DCR): 84.9%.
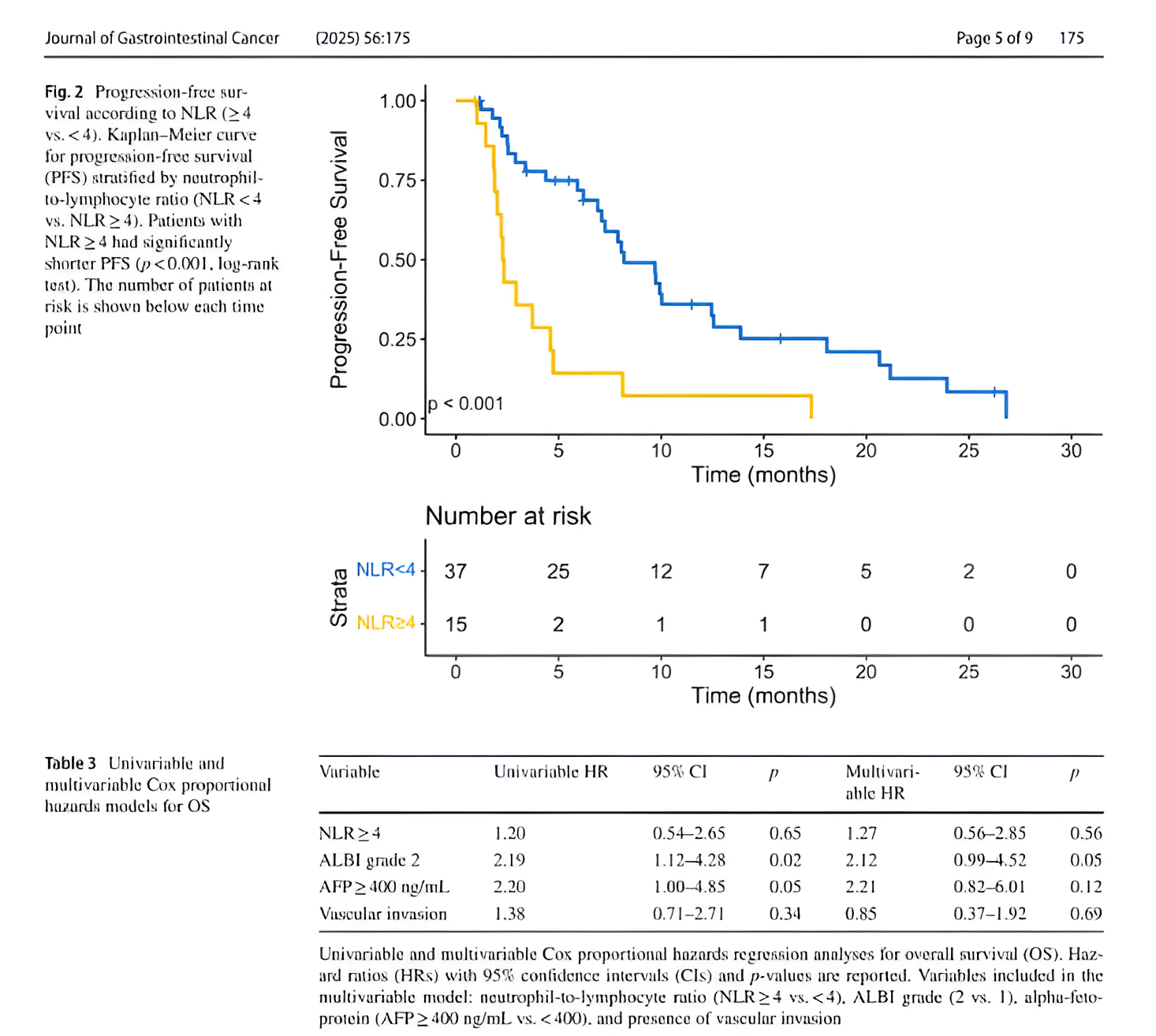
Impact of NLR:
- Median PFS was 2.3 months for patients with NLR ≥ 4 compared to 8.2 months for NLR < 4 (p < 0.001).
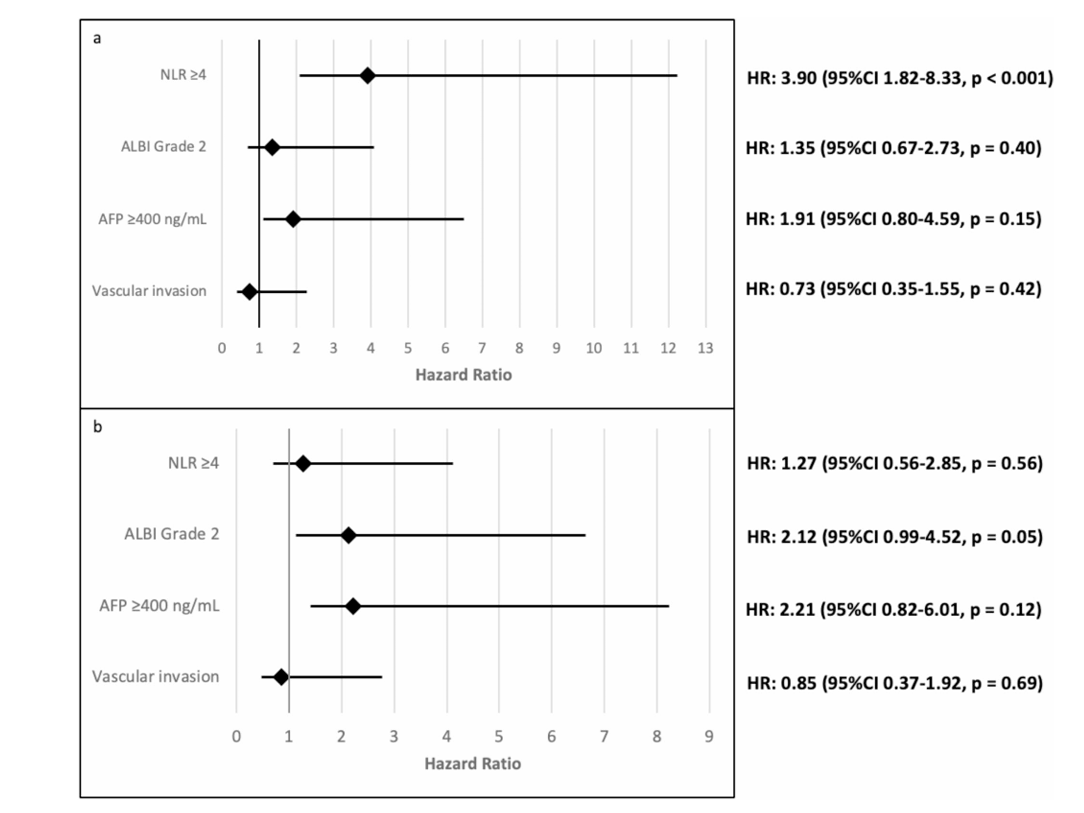
- In multivariable analysis, NLR ≥ 4 was independently associated with shorter PFS (HR 3.90; 95% CI, 1.83–8.33; p < 0.001).
- Patients with NLR ≥ 4 had significantly reduced odds of achieving disease control (OR 0.04; 95% CI, 0.002–0.28; p = 0.005).
- NLR did not significantly correlate with OS (HR 1.27; 95% CI, 0.56–2.85; p = 0.56).
- ALBI grade 2 was the only factor independently associated with worse OS (HR 2.12; 95% CI, 0.99–4.52; p = 0.05)
Key Findings
- Elevated baseline NLR (≥ 4) strongly predicted inferior progression-free survival and lower disease control rates in patients treated with first-line immunotherapy.
- Median PFS dropped from 8.2 months in low-NLR patients to only 2.3 months in high-NLR patients.
- While NLR was predictive for PFS and DCR, it was not associated with overall survival, suggesting later-line treatments may mitigate its effect on long-term outcomes.
- The study population, predominantly non-viral HCC (72.4%), adds valuable data to a field previously dominated by viral-HCC evidence.
- NLR offers a simple, inexpensive, and widely available biomarker that could help identify patients at higher risk of early progression on immunotherapy
Key Takeaway Messages
- NLR ≥ 4 is a strong predictor of poor early outcomes in advanced HCC treated with atezolizumab–bevacizumab or durvalumab–tremelimumab.
- This biomarker could be incorporated into clinical decision-making to stratify patients and trigger early treatment reassessment.
- Unlike OS, which may be influenced by subsequent therapies such as TKIs, PFS and disease control appear more directly affected by baseline inflammatory status.
- The findings support the importance of exploring host immune-inflammatory markers in guiding immunotherapy strategy.
- Further multicenter, prospective studies are warranted to validate NLR across diverse geographic and etiologic HCC populations
Conclusion
This Latin American real-world study demonstrates that baseline NLR is an accessible and cost-effective predictive biomarker for immunotherapy outcomes in advanced HCC. Patients with NLR ≥ 4 had markedly shorter progression-free survival and lower rates of disease control, though overall survival was not significantly impacted. Importantly, the study expands the evidence base to a predominantly non-viral cohort, addressing an underrepresented population in HCC research. Incorporating NLR into clinical practice could help clinicians identify patients less likely to benefit from first-line immunotherapy and prompt earlier consideration of alternative strategies.
You can read the full article here.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
