New Paper Alert: BRCA1/2 Mutations and RB1 Alterations Predict Poorer Outcomes With Endocrine Therapy ± CDK4/6 Inhibitors in HR+/HER2– Breast Cancer
BRCA1/2 mutations, present in approximately 4–5% of hormone receptor–positive/human epidermal growth factor receptor-2–negative (HR+/HER2–) breast cancers, are increasingly recognized as clinically relevant biomarkers influencing treatment response. These tumor suppressor genes play a central role in homologous recombination repair, and their loss leads to genomic instability and potentially reduced benefit from standard endocrine therapy (ET) and cyclin-dependent kinase 4/6 inhibitors (CDK4/6i). Emerging data also highlight the role of RB1 loss-of-function, often co-occurring with BRCA2 mutations, as a driver of resistance to CDK4/6i. Evaluating the prognostic and predictive impact of these alterations is essential for refining treatment strategies and identifying patients who may benefit from alternative approaches, such as poly (ADP-ribose) polymerase inhibitors (PARPi).
Title: Impact of BRCA Mutation on Treatment Outcomes of Endocrine Therapy ± CDK4/6 Inhibitors in Hormone Receptor–Positive/Human Epidermal Growth Factor Receptor-2–Negative Breast Cancer: A Systematic Review and Meta-Analysis
Authors:Hamdy A. Azim, MD, Hagar Elghazawy, MD, Kyrillus S. Shohdy, MSc, Shaimaa Lasheen, MD, Mahmoud Elghazawy, MSc, Ramy Mohamed Ghazy, DrPH, Dalia Abdelnasser, MSc, Loay Kassem, MD
Published in JCO, August 2025
Background
Hormone receptor–positive/human epidermal growth factor receptor-2–negative (HR+/HER2–) breast cancer represents approximately two-thirds of all breast cancer cases. Endocrine therapy (ET) combined with cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) has significantly improved progression-free survival (PFS) and overall survival (OS) compared with ET alone. However, certain genomic alterations, notably RB1 loss-of-function (LOF) and BRCA1/2 mutations, have been implicated in resistance to these treatments.
Germline BRCA1/2 (gBRCA1/2) mutations occur in 4–5% of HR+/HER2– cases and lead to homologous recombination repair (HRR) deficiency. The comparative effectiveness of CDK4/6i versus poly (ADP-ribose) polymerase inhibitors (PARPi) in this subgroup, and the influence of RB1 alterations, remain under investigation.
Methods
A systematic search was conducted in PubMed, Cochrane Library, and Google Scholar from database inception until April 20, 2024, following PRISMA guidelines. Eligible studies included randomized controlled trials, prospective cohorts, and retrospective series reporting DFS, PFS, or OS for HR+/HER2– breast cancer patients receiving ET ± CDK4/6i, stratified by BRCA1/2 or RB1 status. Data from 22 studies (n = 34,960) were included. Hazard ratios (HR) were extracted or calculated from Kaplan–Meier curves. Heterogeneity was assessed using I² and chi-square tests, with fixed-effect or random-effect models applied as appropriate. An additional individual patient analysis was performed using MSK-MET public data.
Study Design
In the early-stage setting, eight studies involving 7,857 patients evaluated adjuvant endocrine therapy outcomes according to BRCA1/2 status, including two randomized controlled trials (MonarchE and SOFT), four retrospective studies, one case–control study, and one prospective observational study, with only MonarchE assessing the combination of endocrine therapy and CDK4/6 inhibitors.
In the metastatic setting, fourteen studies with a total of 27,103 patients examined outcomes with endocrine therapy with or without CDK4/6 inhibitors; eleven studies comprising 12,670 patients analyzed the combination of endocrine therapy and CDK4/6 inhibitors, while two studies involving 14,821 patients evaluated endocrine therapy alone, with palbociclib being the most studied CDK4/6 inhibitor, followed by ribociclib and abemaciclib.
The analysis of RB1 alterations was conducted in four studies including 1,846 patients in the metastatic setting, and all evaluated RB1 status using genomic methods rather than immunohistochemistry.
BRCA1/2 mutation rates ranged from 2.6% to 33% in early BC, and 1.2% to 18% in metastatic cohorts; RB1 alterations occurred in <1–3% of metastatic cases.
Results
Early-stage disease
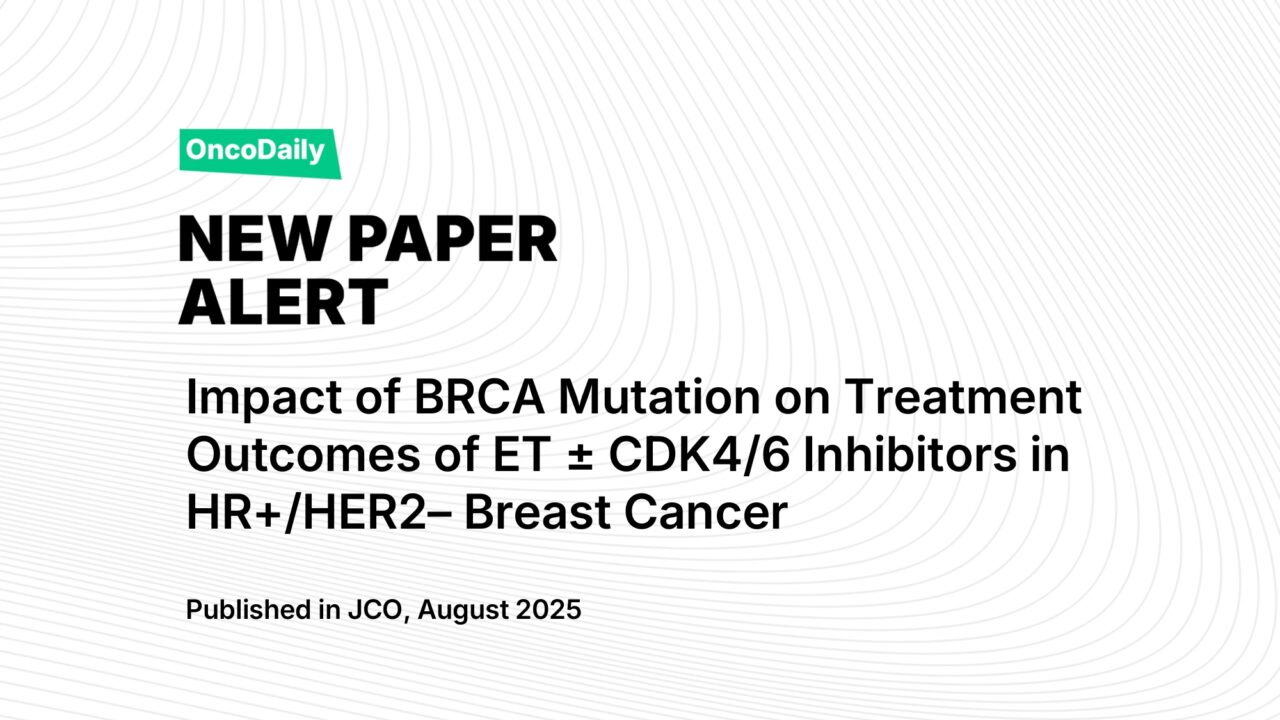
- DFS: Pooled analysis of 5 studies (n = 2,501) showed BRCA1/2 mutation carriers had significantly worse DFS (HR = 1.64; 95% CI, 1.00–2.69; P = .05). Sensitivity analysis excluding one outlier reduced heterogeneity (I² = 7%) and strengthened the association (HR = 2.00; 95% CI, 1.43–2.79; P < .0001).
- OS: 4 studies (n = 5,487) found worse OS for BRCA1/2 mutants (HR = 1.52; 95% CI, 1.20–1.92; P = .0006).
Metastatic disease – ET + CDK4/6i
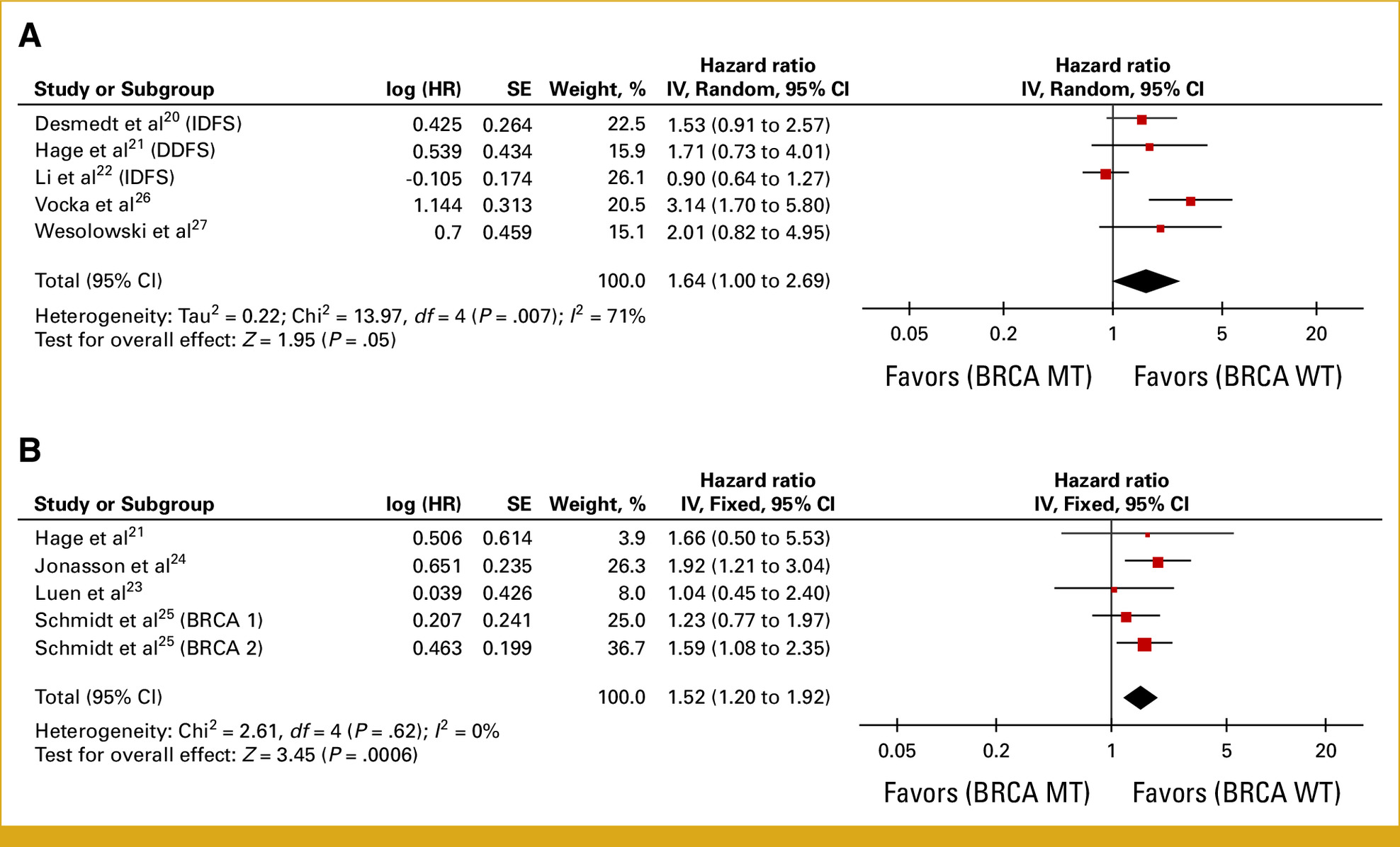
- Any line of therapy: Across 11 studies (n = 12,670), BRCA1/2 mutation carriers had worse PFS (HR = 1.87; 95% CI, 1.45–2.41; P < .00001) and OS (HR = 1.38; 95% CI, 1.15–1.65; P = .0005). After outlier removal, pooled PFS HR improved to 1.40 (95% CI, 1.28–1.54).
- First-line therapy: 4 studies (n = 7,904) showed BRCA1/2 mutants had nearly double the progression risk (PFS HR = 1.99; 95% CI, 1.72–2.30; P < .00001).
- BRCA2-specific: 6 studies (n = 8,155) indicated a more pronounced PFS disadvantage (HR = 2.00; 95% CI, 1.29–3.10; P = .002), driven largely by BRCA2 rather than BRCA1 mutations.
- gBRCA vs tBRCA: Both germline (HR = 1.66; 95% CI, 1.31–2.10) and tumor (HR = 1.47; 95% CI, 1.05–2.07) mutations predicted shorter PFS compared with wild-type.
Metastatic disease – ET alone
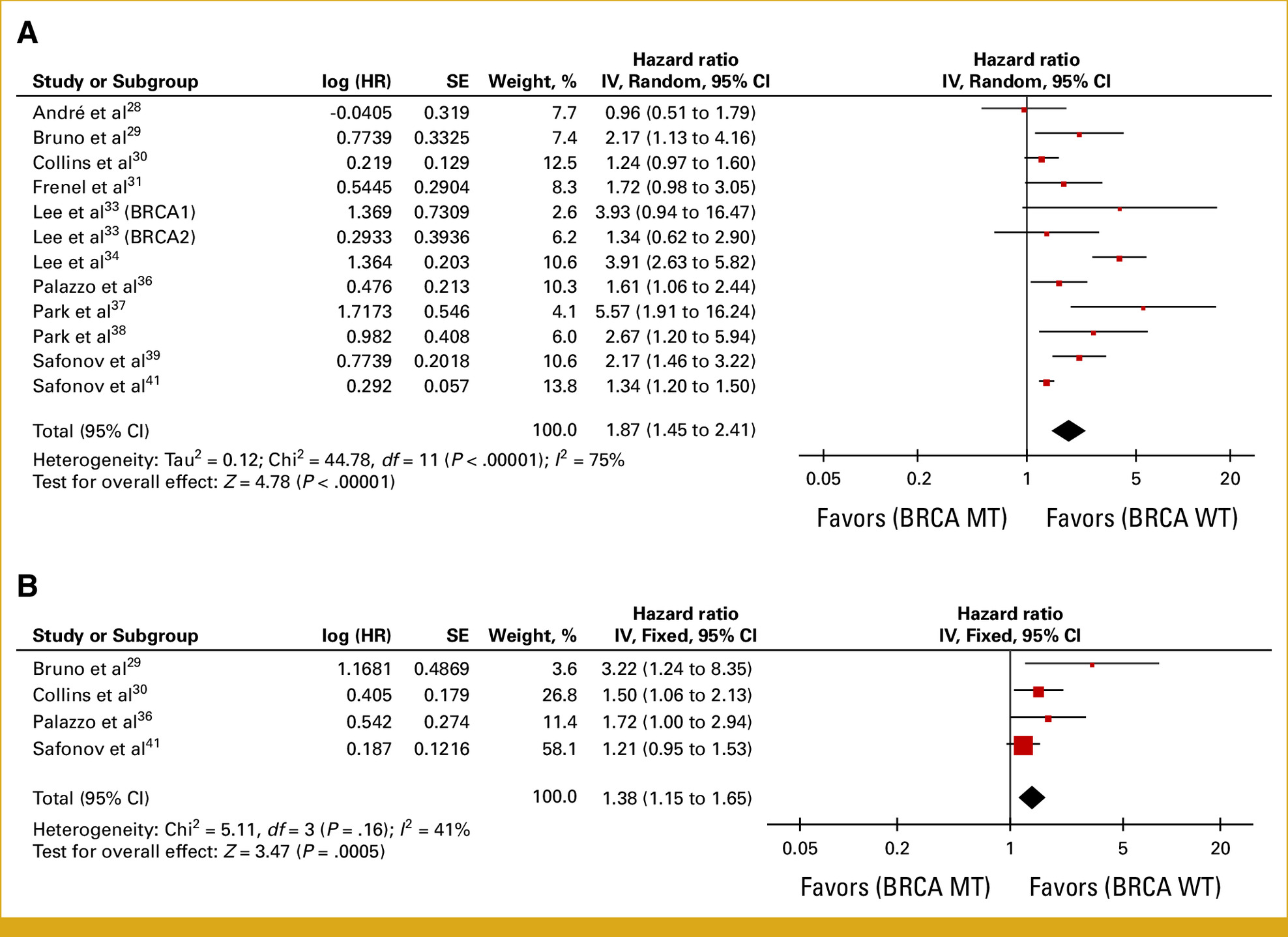
- 2 studies (n = 7,449) found BRCA1/2 mutation carriers had worse PFS (HR = 1.38; 95% CI, 1.19–1.60; P < .0001).
RB1 alterations
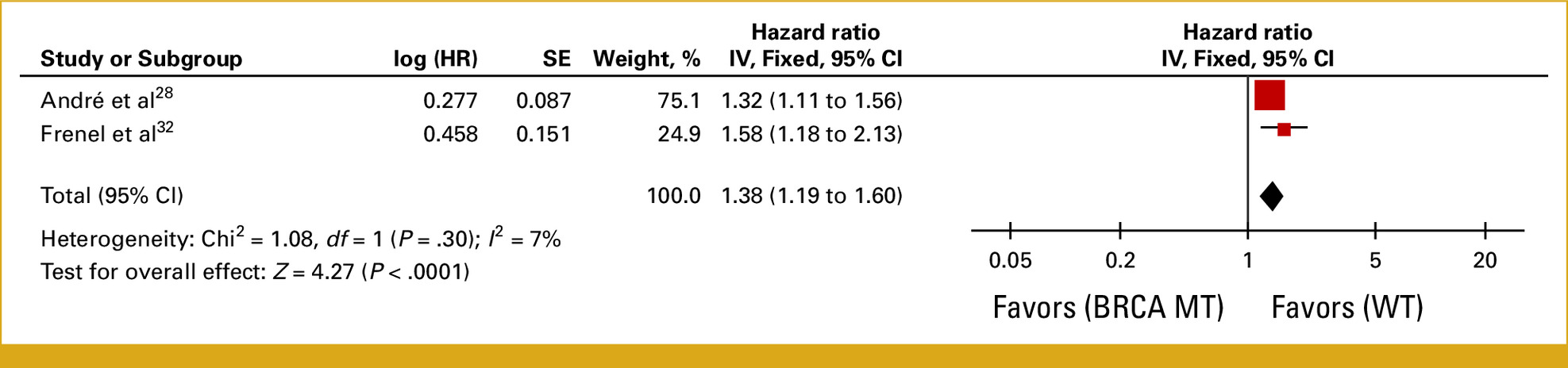
- In 4 studies (n = 1,846), RB1-altered tumors treated with ET + CDK4/6i had significantly worse PFS (HR = 1.85; 95% CI, 1.50–2.27; P < .00001).
- Median PFS in RB1-altered cases was 3.6–3.8 months versus 10–18.9 months in RB1 wild-type.
- Individual patient MSK-MET analysis
- Among 1,897 HR+/HER2– patients, mutation rates were BRCA1: 1.24%, BRCA2: 3.16%, RB1: 2.74%.
- BRCA2 mutations were enriched in metastatic disease (3.7% vs 1.5%; P = .02).
Median OS from metastatic diagnosis:
- BRCA2 mutant: 30 months vs 46 months wild-type (P = .007).
- RB1 altered: 33.2 months vs 46 months wild-type (P = .02).
- BRCA1 mutant: no significant difference (44.4 vs 46 months; P = .59).
Key Findings
Early-stage HR+/HER2– BC:
- BRCA1/2 mutations confer significantly worse DFS and OS with adjuvant ET.
Metastatic setting:
- BRCA1/2 mutations predict worse PFS and OS with ET + CDK4/6i, particularly in first-line therapy.
- The negative impact is stronger in BRCA2-mutated tumors.
- ET alone: Outcomes are also inferior in BRCA1/2 mutants versus wild-type.
- RB1 alterations: Strongly associated with early progression on CDK4/6i, with median PFS reduced to ~3–4 months.
- Genomic co-occurrence: BRCA2 mutations frequently coincide with RB1 loss, potentially driving endocrine resistance.
Key Takeaway Messages
- BRCA2-mutated HR+/HER2– breast cancers may represent a distinct, high-risk luminal subtype with reduced benefit from standard ET ± CDK4/6i.
- RB1 loss compounds resistance to CDK4/6i, warranting genomic testing before treatment planning.
- PARP inhibitors could be a more rational frontline option for gBRCA2-mutated cases, especially when RB1 loss is present.
- Current guidelines still recommend CDK4/6i + ET as first-line therapy irrespective of BRCA status, but accumulating evidence suggests the need for biomarker-guided decisions.
Conclusion
This large meta-analysis of 22 studies and nearly 35,000 patients provides robust evidence that BRCA1/2 mutations—particularly BRCA2—and RB1 alterations predict significantly poorer outcomes with ET ± CDK4/6i in HR+/HER2– breast cancer. The consistent association with reduced PFS and OS, coupled with biological plausibility of endocrine resistance, supports integrating genomic profiling into treatment algorithms. For gBRCA2-mutated patients, especially those with co-existing RB1 loss, frontline PARP inhibitor–based strategies may offer superior disease control. Prospective randomized trials, such as EvoPAR-Breast01, are needed to confirm whether shifting from CDK4/6i to PARPi in this subset can improve long-term survival and redefine standard care.
Access the full article here.
Written by Sona Karamyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
