
FDA Accepts NDA for Vepdegestrant in ESR1-Mutant ER+/HER2- Advanced Breast Cancer
Vepdegestrant is an investigational, orally administered PROteolysis TArgeting Chimera (PROTAC) estrogen receptor degrader, co-developed by Arvinas and Pfizer, designed for the treatment of advanced or metastatic estrogen receptor-positive (ER⁺), HER2-negative (HER2⁻) breast cancer harboring ESR1 mutations. This treatment is intended for patients who have previously received endocrine-based therapy.
The U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA 250810) for vepdegestrant and granted it Fast Track designation, highlighting the urgent need for more effective therapies in this resistant breast cancer subtype. The regulatory submission is primarily supported by results from the VERITAC-2 Phase III trial, which compared vepdegestrant with fulvestrant in this patient population. The FDA has set a Prescription Drug User Fee Act (PDUFA) target action date of June 5, 2026 for its final regulatory decision.
“Patients often face limited treatment options after first-line treatment and vepdegestrant demonstrated improved progression-free survival in patients with ESR1-mutated ER+/HER2- advanced breast cancer.We look forward to working alongside Pfizer and with the FDA to pursue vepdegestrant’s approval and to ensure this important treatment option is made available to patients as rapidly as possible.”
Said John Houston, Ph.D., Chairperson, Chief Executive Officer, and President at Arvinas.
How Vepdegestrant Works
Vepdegestrant is a next-generation PROTAC estrogen receptor degrader designed to target and degrade the estrogen receptor (ER), specifically the mutant forms of the receptor, such as those with ESR1 mutations. It is a novel oral therapy developed to address endocrine resistance in estrogen receptor-positive (ER⁺), HER2-negative (HER2⁻) breast cancer.
Vepdegestrant works by utilizing the PROTAC (PROteolysis TArgeting Chimera) technology, which is a unique strategy to degrade specific proteins inside cells. The PROTAC molecule binds to both the estrogen receptor (ER) and an E3 ubiquitin ligase, facilitating the ubiquitination and subsequent proteasomal degradation of the estrogen receptor. By targeting the estrogen receptor for destruction, vepdegestrant inhibits the signaling pathways that drive cancer cell growth and proliferation in ER-positive cancers.
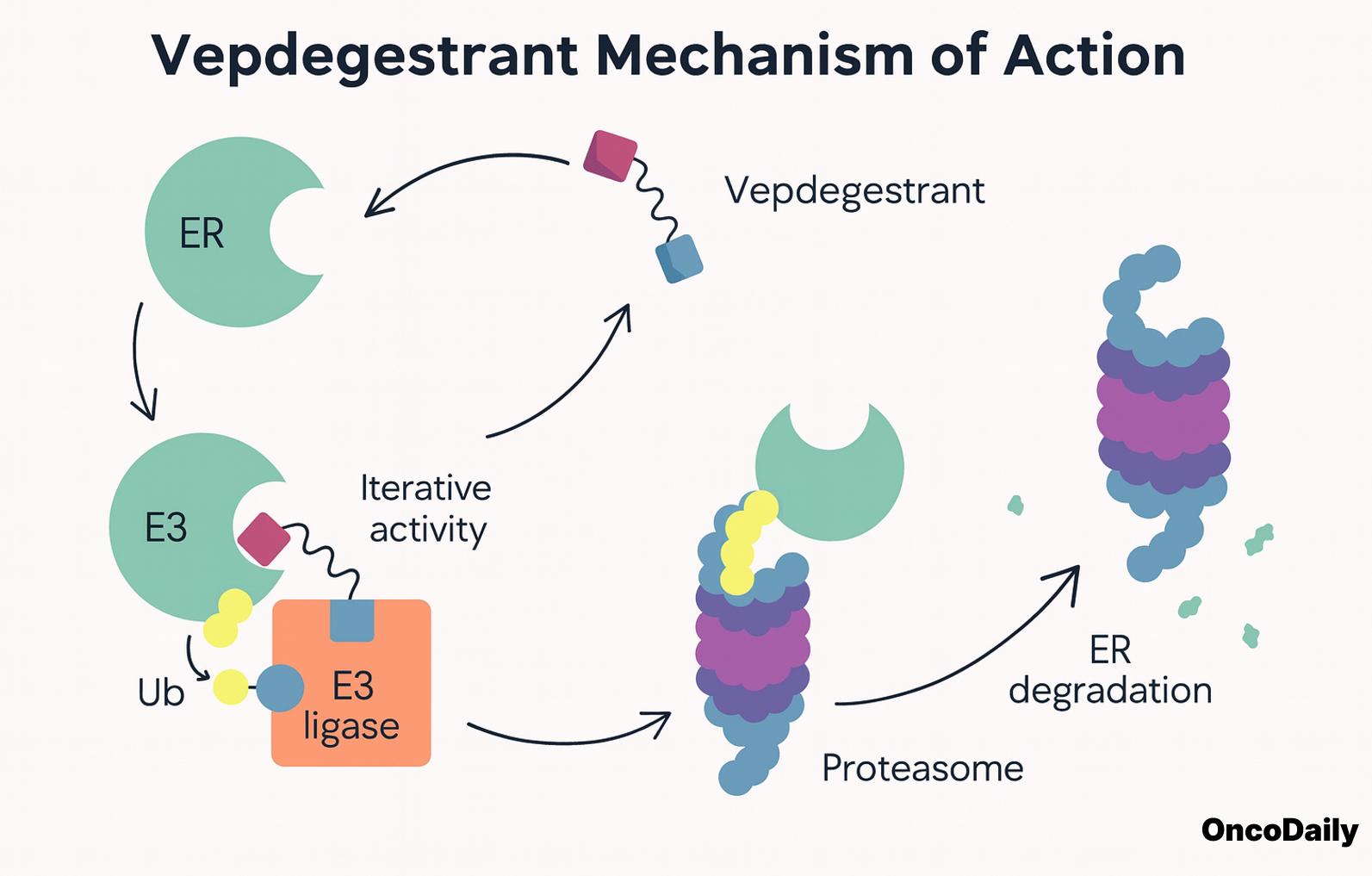
Unlike traditional therapies that simply block the receptor, vepdegestrant is designed to degrade the receptor completely, making it more effective in overcoming the resistance that often develops with conventional endocrine treatments. It is particularly promising for ESR1 mutant breast cancers, where typical estrogen receptor antagonists lose their efficacy.
Vepdegestrant is being developed for patients with advanced or metastatic ER-positive and HER2-negative breast cancer who have developed resistance to prior endocrine therapies, especially those with ESR1 mutations. This mutation is a common mechanism of resistance in ER⁺ breast cancer, which makes vepdegestrant an important potential treatment option in this setting.
The VERITAC-2 Phase III Clinical Trial
The VERITAC-2 study is a randomized, open-label, Phase III clinical trial that focuses on patients with ER+/HER2− advanced breast cancer who harbor ESR1 mutations and have previously received endocrine therapy and CDK4/6 inhibitors. The trial compares the efficacy and safety of two treatment options: vepdegestrant, administered orally at a dose of 1 mg once daily, and fulvestrant, given at a dose of 500 mg intramuscularly on Days 1, 15, and 29 of Cycle 1, followed by Day 1 of each subsequent 28-day cycle. The primary endpoint of the study is progression-free survival (PFS) in patients with ESR1 mutations. Secondary endpoints include overall survival (OS), PFS in the overall patient population, and the assessment of safety profiles of the two treatments.
Study Design and Methods
The VERITAC-2 study is a randomized, open-label, Phase III clinical trial that focuses on patients with ER+/HER2− advanced breast cancer who harbor ESR1 mutations and have previously received endocrine therapy and CDK4/6 inhibitors. The trial compares the efficacy and safety of two treatment options: vepdegestrant, administered orally at a dose of 1 mg once daily, and fulvestrant, given at a dose of 500 mg intramuscularly on Days 1, 15, and 29 of Cycle 1, followed by Day 1 of each subsequent 28-day cycle. The primary endpoint of the study is progression-free survival (PFS) in patients with ESR1 mutations. Secondary endpoints include overall survival (OS), PFS in the overall patient population, and the assessment of safety profiles of the two treatments.
Interventions:
- Vepdegestrant (1 mg orally once daily)
- Fulvestrant (500 mg intramuscularly on Days 1, 15, and 29 of Cycle 1, then Day 1 of each subsequent 28-day cycle)
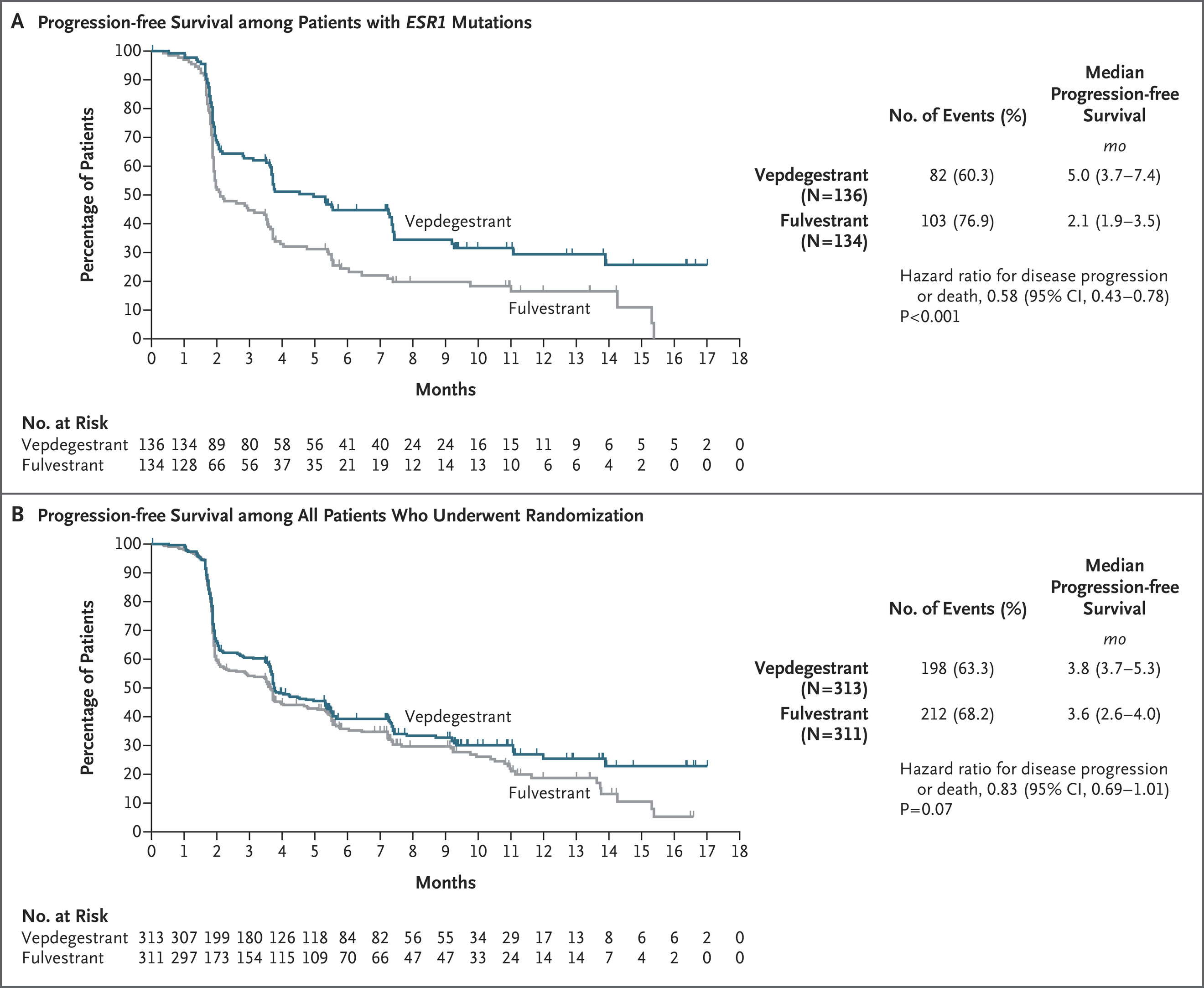
Progression-free Survival as Assessed by Blinded Independent Central Review.
Key Findings
In the Phase III VERITAC-2 trial, vepdegestrant significantly improved progression-free survival (PFS) compared with fulvestrant in patients with ESR1-mutated ER-positive, HER2-negative advanced breast cancer previously treated with endocrine therapy and a CDK4/6 inhibitor. Median PFS in the ESR1-mutated subgroup was 5.0 months versus 2.1 months (HR, 0.58; 95% CI, 0.43–0.78; P<0.001), with higher six-month PFS rates (45.2% vs. 22.7%) and an objective response rate of 18.6% compared with 4.0% for fulvestrant. No statistically significant difference was observed in the overall population (3.8 vs. 3.6 months; HR, 0.83; P=0.07), underscoring the value of biomarker-driven therapy selection.
Vepdegestrant was generally well tolerated, with most adverse events being grade 1–2, including fatigue (26.6%), mild liver enzyme elevations (~14%), and nausea (13.5%). Grade ≥3 events occurred in 23.4% of patients, and permanent discontinuations were rare (2.9%). QTc prolongation was infrequent and not associated with arrhythmias. These findings support vepdegestrant as the first PROTAC therapy to demonstrate Phase III benefit in breast cancer, offering a targeted, oral option for patients with ESR1-mutated disease in the post-CDK4/6 setting.
What Does FDA Fast Track Designation Mean?
Fast Track designation is an FDA program designed to expedite the review of drugs that address serious or life-threatening conditions and fill an unmet medical need. It allows for more frequent communication with the FDA, potential rolling review of application sections, and eligibility for accelerated approval or priority review if certain criteria are met. For patients with ESR1-mutated breast cancer, this designation could bring vepdegestrant to the clinic faster.
What This Could Change in Breast Cancer Treatment?
If approved, vepdegestrant could become the first PROTAC-based therapy available for breast cancer, offering an oral alternative to injectable SERDs for patients with ESR1 mutations. This may change second-line treatment standards, particularly for patients with disease progression after CDK4/6 inhibitors and prior endocrine therapy. By directly degrading the estrogen receptor, it addresses a key resistance mechanism and could extend progression-free survival compared to current options.
The FDA is expected to decide on vepdegestrant’s approval by June 5, 2026. If granted, it would represent a significant milestone for targeted protein degradation therapies and potentially reshape treatment strategies for ER+/HER2- metastatic breast cancer.
You can read the full article here.

Written by Sona Karamyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023

