Real-World Study Confirms Nivolumab Plus Cabozantinib Improves Survival in Metastatic RCC
Original Study Title: Nivolumab plus cabozantinib in metastatic renal cell carcinoma: real-world evidence from the international ARON-1 study
Authors: Maria T. Bourlon, Luca Galli, Enrique Grande, Se Hoon Park, Bohuslav Melichar, Timothy J. Schieber, Maria José Juan-Fita, Yüksel Ürün, Javier Molina-Cerrillo, Teresa Alonso-Gordoa, Ugo De Giorgi, Jakub Kucharz, Esther Pérez Calabuig, Vincenza Conteduca, Tarek Taha, Pasquale Rescigno, Hussam Abu-Sini, Gian Paolo Spinelli, Ray Manneh Kopp, Alessia Salfi, Dipen Bhuva, Paola Valdez-Sandoval, Sofia Mendez-Bribiesca, Ondrej Fiala, Sebastiano Buti, Fernando Sabino Marques Monteiro, Aristotelis Bamias, Marwan Ghosn, Francesco Massari, Jawaher Ansari, Matteo Santoni
Published in: Frontiers in Oncology, July 2025
This article presents real-world evidence on the effectiveness and safety of nivolumab plus cabozantinib as a first-line treatment for metastatic renal cell carcinoma (mRCC). Based on the international ARON-1 study, this retrospective analysis includes 333 patients treated across 52 centers in 17 countries. The findings confirm that the survival and response benefits seen in clinical trials are reproducible in daily practice, even among patients typically underrepresented in trials—such as those with ECOG ≥2, non-clear cell histology, sarcomatoid features, or brain metastases.
At a median follow-up of 15.9 months, the two-year overall survival rate reached 75%, with a median progression-free survival of 33.7 months. The combination was well tolerated, with a manageable toxicity profile, supporting its role as a standard first-line option in diverse clinical settings.
Introduction
The emergence of immune checkpoint inhibitors (ICIs), both as monotherapy and in combination with VEGFR tyrosine kinase inhibitors (VEGFR-TKIs), has revolutionized the treatment of advanced renal cell carcinoma (RCC). Regimens such as nivolumab plus ipilimumab, pembrolizumab plus axitinib, and nivo plus cabozantinib have shown superior outcomes in pivotal phase III trials, significantly improving overall survival (OS), progression-free survival (PFS), and response rates, while maintaining acceptable toxicity profiles. The CheckMate 9ER trial demonstrated that the combination of nivo and cabozantinib reduced the risk of death by nearly 40% and improved both median PFS and overall response rate (ORR) compared to sunitinib.
However, the applicability of these findings in the broader clinical population remains a concern, as randomized trials often exclude patients with poor performance status or complex disease characteristics. Real-world evidence (RWE) can complement clinical trial data by including such underrepresented populations, assessing long-term outcomes, late toxicities, and treatment patterns across diverse clinical settings.
In this context, the ARON-1 study aimed to assess the real-world effectiveness and safety of first-line nivolumab plus cabozantinib in patients with metastatic RCC (mRCC). This retrospective, multicenter analysis includes data from 52 centers across 17 countries in Europe, Asia, and the Americas, representing the largest international cohort to date for this regimen outside a clinical trial setting.
Methods and Study Design
This retrospective cohort included adult patients (≥18 years) with histologically confirmed RCC and radiologically or histologically confirmed metastatic disease. Eligible patients were treated with first-line nivolumab plus cabozantinib between January 1, 2021, and September 1, 2024. Nivolumab was administered intravenously every 2 or 4 weeks, and cabozantinib was given orally once daily, with dosing adjusted based on physician discretion and patient tolerance.
Tumor assessments were conducted via contrast-enhanced CT or MRI every 8–12 weeks, with laboratory and physical evaluations performed at 4–6 week intervals. Tumor response was assessed per RECIST 1.1 criteria. The primary endpoints included overall survival (OS), progression-free survival (PFS), and objective response rate (ORR), with duration of response (DOR) calculated among responders. Subgroup analyses and landmark analyses at 6 and 12 months were conducted to adjust for follow-up differences.
Adverse events were graded according to CTCAE v5.0. Patients who did not experience progression or were lost to follow-up were censored at their last available visit. Statistical analysis was performed using MedCalc software, with Kaplan-Meier curves, Cox proportional hazards modeling, and appropriate comparative tests for categorical variables. All statistical tests were two-sided, with significance set at p < 0.05.
Results
Patient Population:
- Total: 333 patients
- Median age: 63 years
- Clear cell histology: 82%
- Sarcomatoid features: 12%
- Prior nephrectomy: 60%
- IMDC risk: 18% good, 54% intermediate, 28% poor
- Common metastases: lung (60%), lymph nodes (49%), bone (32%), liver (13%), brain (6%)
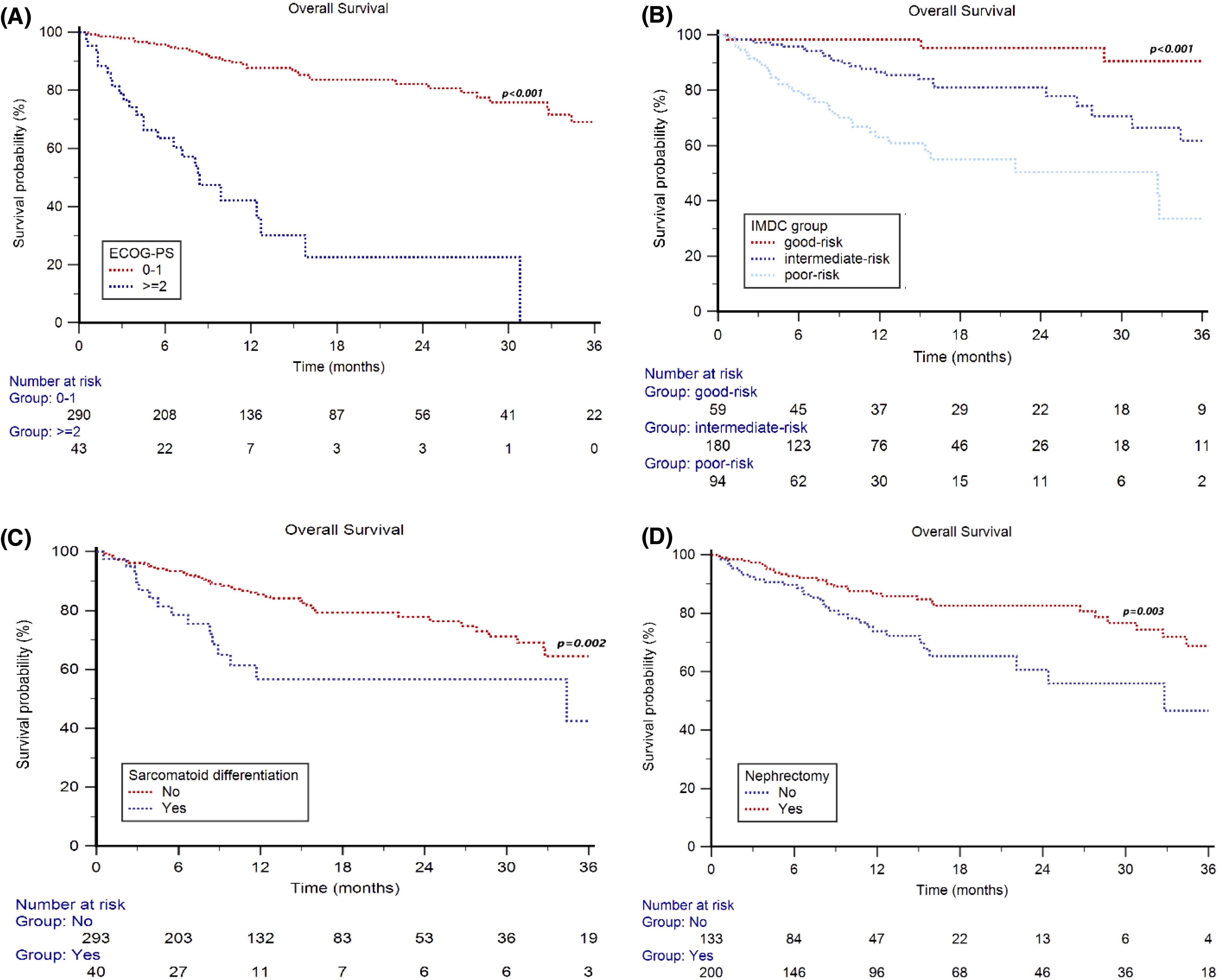
Overall Survival (OS):
- Median OS: Not reached
- 2-year OS rate: 75% overall
- By ECOG: 82% (0–1) vs 23% (≥2)
- By IMDC: 95% (good), 81% (intermediate), 50% (poor)
- Sarcomatoid histology: 2y-OS = 57% vs 78%
- Prior nephrectomy: 83% (yes) vs 61% (no)
- Brain metastases: 2y-OS = 35% vs 77%
Progression-Free Survival (PFS):
- Median PFS: 33.7 months
- Landmark PFS at 12 months: 41.4 months
- Second-line therapy PFS: 5.3 months
Response to Therapy:
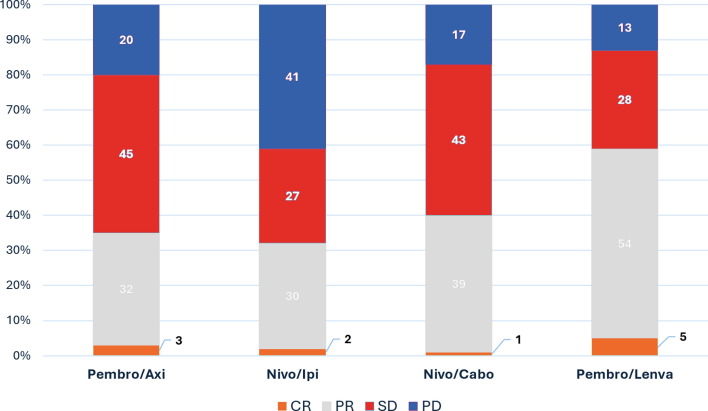
- Overall Response Rate (ORR): 58%
- CR: 6%, PR: 52%, SD: 31%, PD: 11%
- Median Duration of Response (DOR): 38.9 months
Prognostic Factors (Multivariate):
- ECOG ≥2, sarcomatoid differentiation, bone/brain metastases, and poor IMDC risk independently associated with worse OS
Safety Profile:
- Grade 3–4 AEs: 37%
- Common severe AEs: hypertension (9%), hand-foot syndrome (8%), diarrhea (7%), fatigue (7%)
- Cabozantinib dose reduction: 32%
- Treatment discontinuation due to AEs: cabozantinib (22%), nivo (12%)
- Dose adjustments did not impact OS
Key Findings
The ARON-1 study offers the most comprehensive real-world evaluation to date of first-line nivo plus cabozantinib in patients with metastatic renal cell carcinoma (mRCC), confirming the clinical benefit of this combination across a broad and heterogeneous patient population. The findings revealed that the overall survival benefit observed in the phase III CheckMate 9ER trial translates well into clinical practice, with a two-year OS rate of 75% and median OS not reached. Notably, survival benefits were consistent across age and sex groups.
Prognostically, patients with favorable characteristics—such as ECOG performance status 0–1, prior nephrectomy, clear cell histology, and absence of metastases to the bone, liver, or brain—experienced significantly improved outcomes. Conversely, patients with ECOG ≥2, sarcomatoid differentiation, IMDC poor-risk status, or brain metastases showed substantially worse survival, reaffirming their roles as poor prognostic indicators.
Treatment response was robust in both clear cell and non-clear cell histologies, with an overall response rate of 58%, and the median duration of response reaching nearly 39 months. Importantly, this regimen also yielded meaningful responses in poor-risk and non-clear cell subgroups, traditionally underrepresented in clinical trials.
Progression-free survival was notably prolonged, with a median PFS of 33.7 months—substantially longer than the PFS reported in clinical trials. This may reflect differences in real-world imaging intervals, RECIST interpretation, and continuation of treatment beyond progression.
Safety outcomes were favorable, with a lower incidence of grade ≥3 adverse events compared to trial data. Common toxicities included hypertension, hand-foot syndrome, and diarrhea, all manageable in most cases. Dose reductions and treatment interruptions were frequent but did not compromise overall survival, indicating that this regimen is tolerable and adaptable to individual patient needs in routine care.
Key Takeaway Messages
-
Nivolumab plus cabozantinib demonstrated durable survival and response outcomes in a real-world setting, closely aligning with phase III trial results.
-
The 2-year overall survival rate was 75%, and the median OS was not reached.
-
Efficacy was consistent across age and sex, but diminished in patients with ECOG ≥2, sarcomatoid histology, and poor-risk IMDC classification.
-
Patients with bone, liver, or brain metastases had significantly shorter OS.
-
The ORR was 58%, with higher rates observed in patients with good-risk IMDC and clear cell histology.
-
Median PFS reached 33.7 months, exceeding clinical trial benchmarks.
-
Safety profile was favorable, with lower-than-expected rates of grade ≥3 toxicities.
-
Dose reductions and AE-related treatment interruptions did not negatively affect survival outcomes.
- This real-world study confirms the broad applicability and effectiveness of nivolumab plus cabozantinib across diverse healthcare systems and patient populations.
Conclusion
The ARON-1 study provides compelling real-world evidence supporting the effectiveness and safety of nivolumab plus cabozantinib as a first-line treatment for metastatic renal cell carcinoma (mRCC). With data collected from 333 patients across 52 centers in 17 countries, this retrospective multicenter analysis confirms that the survival and response outcomes observed in controlled clinical trials, such as CheckMate 9ER, are largely reproducible in routine clinical practice.
Notably, the combination therapy achieved durable responses and prolonged progression-free survival, even in subgroups traditionally excluded from clinical trials, such as patients with ECOG performance status ≥2, brain metastases, or non-clear cell histologies. The findings highlight the regimen’s broad applicability across a diverse, global patient population.
Furthermore, the tolerability profile was favorable, with a lower incidence of high-grade adverse events compared to phase III data. Importantly, dose modifications and treatment interruptions due to toxicity did not negatively impact survival outcomes, underscoring the regimen’s feasibility in real-world settings.
Incorporating these real-world insights into clinical decision-making can enhance the personalization of mRCC management, especially when treating frail patients or those with aggressive disease features. The ARON-1 study reinforces the role of nivo plus cabozantinib as a versatile and effective frontline option and highlights the value of real-world data in complementing clinical trial findings to guide oncologic care in daily practice.
You can Read The Full Article on Frontiers in Oncology
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
