Telisotuzumab vedotin (Emrelis) Updates 2025: Uses in Cancer, Dosage, Side Effects, Expectations, and More
Telisotuzumab vedotin is an innovative antibody-drug conjugate (ADC) designed to target and attack cancer cells with precision. It combines a monoclonal antibody that binds to a specific protein on the surface of certain cancer cells with a powerful chemotherapy agent. This targeted approach allows the drug to deliver its anti-cancer effects more directly, potentially reducing harm to healthy cells and improving treatment outcomes. This article will help you explore Telisotuzumab vedotin’s dosage, side effects, what to expect during treatment, and how it is being investigated for use in cancer care.
Which company produced Telisotuzumab vedotin?
Telisotuzumab vedotin, marked as Emrelis, is produced by AbbVie – the biopharmaceutical giant behind Telisotuzumab vedotin (also known as ABBV‑399) – a global company headquartered in North Chicago, Illinois. AbbVie is widely recognized for its strong pipeline and expertise in oncology, with a focus on developing targeted therapies that address serious and complex diseases. Telisotuzumab vedotin is an antibody-drug conjugate (ADC) that combines a monoclonal antibody targeting the c‑Met receptor with a powerful chemotherapy agent, monomethyl auristatin E (MMAE), aiming to deliver treatment directly to cancer cells while sparing healthy tissue.
AbbVie has guided this therapy through early research and clinical development as part of its broader commitment to advancing precision oncology. In developing this ADC, AbbVie also collaborates with Seagen (formerly Seattle Genetics), a leader in ADC technology. This partnership allows AbbVie to incorporate Seagen’s established MMAE-based linker-payload system—the same technology used in other well-known ADCs like brentuximab vedotin and enfortumab vedotin—helping to enhance the precision and potency of its cancer therapies.
How does Telisotuzumab vedotin Work?
Telisotuzumab vedotin is designed with precision in mind. At its core, it combines the targeting power of a monoclonal antibody with the strength of a chemotherapy drug. The antibody portion of the drug is trained to recognize and attach to a specific protein called c-Met, which is found in high amounts on the surface of certain cancer cells. In normal cells, c-Met plays a role in cell growth and repair, but when overexpressed in tumors, it can drive cancer progression and spread.
Once Telisotuzumab vedotin binds to c-Met, it is absorbed into the cancer cell. Inside, it releases its hidden weapon—monomethyl auristatin E (MMAE)—a potent chemotherapy agent. MMAE works by disrupting the cell’s internal structure, stopping it from dividing and ultimately causing the cancer cell to die. By combining targeted delivery with powerful treatment, Telisotuzumab vedotin aims to destroy cancer cells while limiting damage to healthy tissue, making it a promising option in the growing field of antibody-drug conjugates.
What Cancers are treated with Telisotuzumab vedotin?
On May 14, 2025, Emrelis (telisotuzumab vedotin-tllv) received accelerated approval from the U.S. FDA. Developed by AbbVie, the drug is now authorized for adults with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) that shows high c-Met protein overexpression—defined as strong expression in at least 50% of tumor cells. This approval applies to patients who have already received prior systemic therapy.
What research is behind the approval?
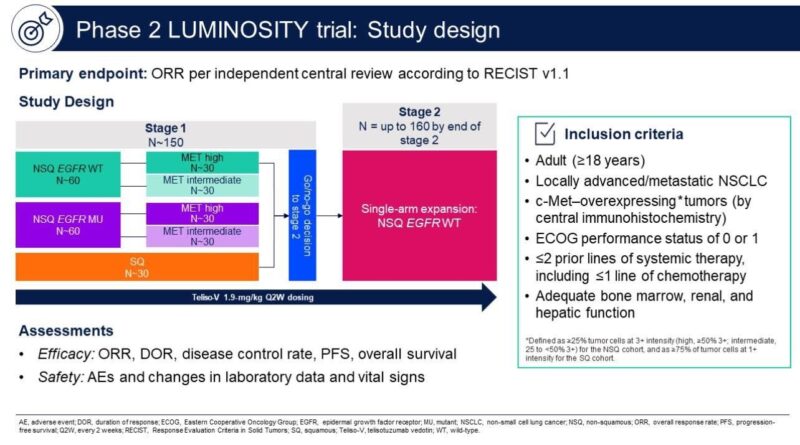
The FDA’s accelerated approval of Emrelis (telisotuzumab vedotin-tllv) was based on findings from the pivotal Phase 2 LUMINOSITY trial (NCT03539536), which investigated the drug as a monotherapy in patients with previously treated, c-Met–overexpressing, non-squamous, EGFR wild-type advanced non-small cell lung cancer (NSCLC). The trial aimed to identify the subgroup of patients most likely to benefit from this targeted therapy and focused on those with moderate to high levels of c-Met protein expression, a biomarker associated with aggressive tumor behavior and poor prognosis.
The study enrolled 172 patients, and 161 of them were included in the primary efficacy analysis. Among the 78 patients whose tumors had high c-Met overexpression (defined as ≥50% of tumor cells showing strong staining), the drug achieved an overall response rate (ORR) of 34.6%, with a median duration of response (DOR) of 9 months. In patients with intermediate c-Met expression (25% to <50%), the ORR was 22.9%, with a median DOR of 7.2 months. The overall response rate across all participants was 28.6%. Importantly, the majority of responses lasted 6 months or longer, reflecting meaningful and durable clinical benefit in this hard-to-treat population.
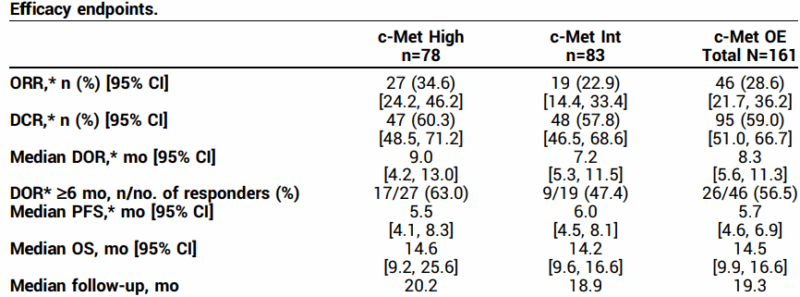
The safety profile was consistent with known effects of antibody-drug conjugates. The most frequently reported treatment-related side effects included peripheral sensory neuropathy (30%), peripheral edema (16%), and fatigue (14%). Two patients experienced serious grade 5 adverse events (interstitial lung disease and respiratory failure), but overall, the side effects were considered clinically manageable and in line with prior expectations.
These promising results were presented during a Special Clinical Science Symposium at the 2024 ASCO Annual Meeting and published in the Journal of Clinical Oncology (Volume 42, Supplement 16). The trial’s findings played a key role in supporting the FDA’s decision to grant accelerated approval to Emrelis for adult patients with previously treated, locally advanced or metastatic non-squamous NSCLC with high c-Met protein overexpression.
You can read more about Telisotuzumab Vedotin (EMRELIS™) Approval for c-MET–Overexpressing Advanced Non-Small Cell Lung Cancer on OncoDaily.
Telisotuzumab vedotin Side Effects and Its Management
Telisotuzumab vedotin, while offers important benefits, can cause several side effects. Knowing what to expect and how to manage these side effects can help patients stay on treatment and maintain quality of life.
Common Side Effects of Telisotuzumab Vedotin
Many patients treated with Emrelis experience side effects such as low albumin levels and high blood sugar. Peripheral neuropathy, which causes tingling, numbness, or burning sensations in the hands and feet, is also a common side effect. Other frequently reported issues include fatigue, swelling (peripheral edema), decreased appetite, nausea, and constipation. Changes in liver enzymes, electrolyte imbalances (including calcium, sodium, potassium, and magnesium), low blood counts, kidney function changes, blurred vision, and eye inflammation (keratitis) may also occur.
Less Common but Serious Side Effects
Severe peripheral neuropathy can develop in some patients and may significantly affect daily activities. This serious nerve damage requires close monitoring and may necessitate adjusting or stopping treatment.
How to Manage Side Effects of Telisotuzumab Vedotin?
Managing peripheral neuropathy is essential. Patients should report early symptoms like numbness or tingling so healthcare providers can adjust treatment to prevent worsening. Medications such as gabapentin or duloxetine can help relieve nerve pain. Supportive care is important for other side effects. Nutritional support can help with low albumin levels and loss of appetite. Electrolyte imbalances need correction to avoid complications. Fatigue can be managed by balancing rest and activity and addressing any other medical issues.
Swelling can be reduced by elevating the legs and using compression stockings. Regular monitoring of liver function is necessary, with treatment adjustments if needed. Nausea and constipation usually respond well to anti-nausea medications and laxatives.Patients should be closely observed for infections, including pneumonia, which requires prompt treatment. Any eye symptoms like keratitis should be evaluated by an eye specialist quickly.
What is the Recommended Dosage of Telisotuzumab vedotin?
Telisotuzumab vedotin is supplied as a lyophilized powder that comes in two strengths: 20 mg and 100 mg. It is intended for use in adults with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) that shows high c-MET protein overexpression—defined as strong staining (3+) in at least 50% of tumor cells. This treatment is used in patients who have already received one prior systemic therapy.
The recommended dose is 1.9 mg per kilogram of body weight, given every two weeks. In patients who weigh 100 kg or more, the dose should not exceed 190 mg. Treatment is continued until the cancer progresses or the side effects become unmanageable.
Learn more about Non-Small Cell Lung Cancer: Causes, Symptoms, Diagnosis, Treatment Options, and Latest 2025 Advances in Targeted and Immunotherapy on OncoDaily.
How is Telisotuzumab vedotin administered?
Telisotuzumab vedotin is compatible with 0.9% sodium chloride and requires careful preparation due to its hazardous components. Handling and disposal must follow local safety guidelines.
Before use, the lyophilized powder should reach room temperature. Sterile water for injection is added slowly to reconstitute the powder—1.1 mL for the 20 mg vial and 5.2 mL for the 100 mg vial—resulting in a final concentration of 20 mg/mL. The vial should be gently swirled until fully dissolved (no shaking), and the solution must be clear to slightly yellow and free from particles.
After reconstitution, the correct dose is withdrawn and added to a saline infusion bag to achieve a concentration of 1–10 mg/mL. The bag is gently inverted to mix and checked for clarity. Unused vial contents must be discarded. Premedication is recommended for patients who had previous infusion reactions and typically includes an antihistamine, antipyretic, and corticosteroid given 30–60 minutes before administration.
What to Avoid During Telisotuzumab vedotin Treatment?
During treatment with telisotuzumab vedotin, certain precautions are essential to ensure safety and effectiveness. The drug should be given through a dedicated IV line and should never be mixed with other medications or shaken during preparation, as this can affect its stability.
Patients should not ignore early signs of peripheral neuropathy, such as tingling or numbness, as prompt reporting allows for timely management. Regular lab monitoring is also important to detect issues with liver function, blood counts, or electrolytes—missing these tests can lead to unnoticed complications. Because the immune system may be weakened, patients should avoid exposure to infections and maintain good hygiene. Pregnancy and breastfeeding must also be avoided during and shortly after treatment, due to the risk of harm to the baby. By following these precautions, patients can reduce side effects and stay on track with their treatment plan.
Telisotuzumab vedotin effectiveness over time
Updated results from the LUMINOSITY trial evaluating telisotuzumab vedotin monotherapy in previously treated patients with c-Met protein–overexpressing, EGFR wild-type, nonsquamous non-small cell lung cancer (NSCLC) were published in the Journal of Thoracic Oncology. In this analysis (data cutoff: February 21, 2024), 168 patients (84 with high c-Met expression, 84 with intermediate) received Teliso-V at 1.9 mg/kg every two weeks. The overall response rate (ORR) was:
- 34.5% in the c-Met high group
- 23.8% in the c-Met intermediate group
- 29.2% overall
The median duration of response (DOR) was 7.2 months across all groups, with over half of responders maintaining responses for ≥6 months. Progression-free survival (PFS) was 5.6 months, and overall survival (OS) was 14.2 months. Treatment was generally well tolerated. The most common treatment-related adverse events were peripheral sensory neuropathy (31%), peripheral edema (16%), and fatigue (14%). Grade ≥3 events were infrequent, with sensory neuropathy (7%) being the most common. These updated findings support Teliso-V’s sustained activity and manageable safety profile, particularly in the c-Met high subgroup.
Ongoing trials with Telisotuzumab vedotin
The global Phase 3 trial (NCT06093503), sponsored by AbbVie, is designed to evaluate whether combining telisotuzumab vedotin with osimertinib improves outcomes compared to standard platinum-based chemotherapy in adults with advanced non-squamous non-small cell lung cancer (NSCLC) that has both an EGFR mutation and c-Met protein overexpression. The study focuses on patients whose cancer progressed after treatment with a third-generation EGFR inhibitor.
Around 250 participants will be randomized to receive either telisotuzumab vedotin with daily osimertinib, or standard chemotherapy with cisplatin or carboplatin and pemetrexed. The treatment period includes regular hospital visits, medical assessments, lab tests, and questionnaires. The study is expected to last just under four years.
FAQ
What is Emrelis used for?
Emrelis is used to treat adults with advanced or metastatic non-small cell lung cancer (NSCLC) that shows high levels of a protein called c-Met. It is specifically given to patients whose cancer has already been treated with other systemic therapies.
What does "c-Met overexpression" mean?
c-Met overexpression means that cancer cells produce too much of the c-Met protein. This protein can help cancer cells grow and spread. Telisotuzumab vedotin targets cells with high c-Met levels, making it a personalized treatment option for certain patients.
What are the most common side effects of Telisotuzumab vedotin?
Some of the most common side effects include nerve pain or numbness (peripheral neuropathy), swelling in the legs or arms, tiredness, high blood sugar, and changes in liver or kidney tests.
How is Telisotuzumab vedotin given to patients?
Emrelis is given through an intravenous (IV) infusion in a clinical setting. It is usually administered every two weeks. The dose is based on a patient’s body weight, and preparation must follow special safety handling rules due to the drug’s potency.
Are there ongoing studies involving Telisotuzumab vedotin?
A major Phase 3 trial is testing it with osimertinib in patients whose lung cancer has both an EGFR mutation and high c-Met expression. These studies aim to expand treatment options in the future.
How is Telisotuzumab Vedotin broken down in the body?
After entering the body, Telisotuzumab Vedotin is broken down into smaller parts, including a chemotherapy piece called MMAE. The liver helps process this chemotherapy part through natural enzymes.
How long does Telisotuzumab Vedotin stay in the body?
Telisotuzumab Vedotin stays in the body for a few days. The main drug lasts about 3 days, while the chemotherapy part (MMAE) may stay around 4 days before being cleared from the system.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
