Retifanlimab-dlwr (Zynyz) Updates 2025: Uses in Cancer, Side effects, Dosage, Expectations and more
Zynyz is an investigational humanized IgG1 monoclonal antibody targeting the PD-1 receptor. By blocking the interaction between PD-1 and its ligands (PD-L1/PD-L2), it aims to restore T-cell activity and promote anti-tumor immune responses across a range of malignancies. This article reviews the clinical development of Zynyz, including its therapeutic indications, safety profile, dosing regimen, and treatment expectations.
Which company produced Retifanlimab-dlwr?
Retifanlimab‑dlwr, marked as a brand name Zynyz, is produced and marketed by Incyte Corporation, which holds the U.S. commercial rights. The drug was originally developed by MacroGenics, Inc., and Incyte acquired global rights through a strategic collaboration in 2017.
Incyte Corporation, founded in 2002 and headquartered in Wilmington, Delaware, is a biopharmaceutical company focused on discovering and developing innovative therapies in oncology, inflammation, and autoimmune diseases. The company is known for its successful drugs such as Jakafi® (ruxolitinib) and Opzelura®, and operates globally with research and development sites across North America, Europe, and Asia.
Incyte has also engaged in key partnerships to expand its capabilities and pipeline. Beyond its collaboration with MacroGenics for Zynyz, it has established alliances with companies like Agenus—for the development of novel immune checkpoint antibodies—and Agilent Technologies to develop companion diagnostics for its oncology portfolio. These partnerships reflect Incyte’s broader strategy of advancing targeted and immune-based therapies through both in-house innovation and external collaboration.
How does Retifanlimab-dlwr work?
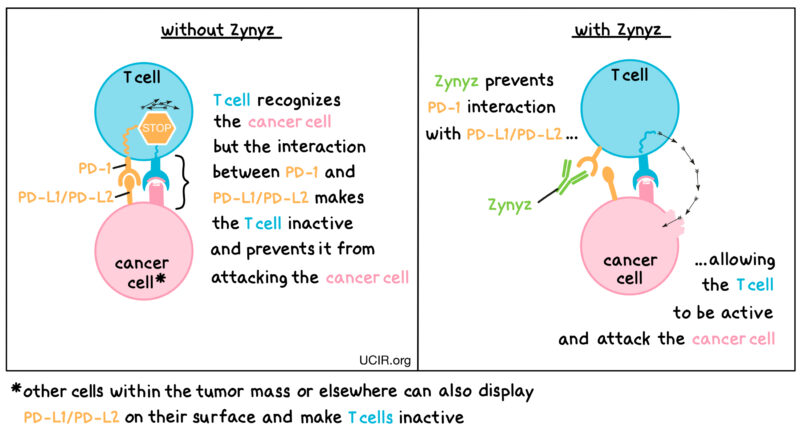
Zynyz (retifanlimab‑dlwr) is an immunotherapy that enhances the body’s immune response against cancer cells. It is a humanized IgG4 monoclonal antibody that targets the programmed death receptor-1 (PD-1) on T cells, a crucial component of the immune system.
Under normal circumstances, the PD-1 receptor on T cells interacts with its ligands, PD-L1 and PD-L2, which are often expressed on tumor cells. This interaction inhibits T cell activation, allowing cancer cells to evade immune detection and destruction. By binding to the PD-1 receptor, Zynyz blocks this interaction, effectively “releasing the brakes” on the immune system. This enables T cells to become active and attack cancer cells more effectively.
What Cancers Is Retifanlimab-dlwr Approved to Treat?
Zynyz (retifanlimab-dlwr) has emerged as a groundbreaking immunotherapy with FDA approvals for multiple difficult-to-treat cancers. In March 2023, it became the first approved treatment for adults with metastatic or recurrent locally advanced Merkel cell carcinoma (MCC), a rare and aggressive skin cancer. This approval marked an important advance, offering new hope to patients facing limited options.
Building on this success, Zynyz received a second FDA approval in May 2025, making it the first and only immunotherapy approved as a first-line treatment for advanced anal cancer in the United States. Specifically, it is authorized for use in combination with chemotherapy for patients with inoperable, locally recurrent, or metastatic squamous cell carcinoma of the anal canal (SCAC). Additionally, Zynyz is approved as a single agent for patients whose disease has progressed after or who cannot tolerate platinum-based chemotherapy.
What research is behind the approval?
The initial FDA approval of Zynyz (retifanlimab-dlwr) for metastatic or recurrent locally advanced Merkel cell carcinoma (MCC) was based on findings from the Phase 2 POD1UM-201 trial (NCT03599713). This open-label, single-arm study enrolled 65 patients who had not received prior systemic therapy for advanced MCC.
Zynyz showed an objective response rate (ORR) of 52%, with 18% achieving a complete response. Notably, most responses were durable: 76% of patients maintained their response for six months or longer, and 62% for at least 12 months, based on independent central review using RECIST v1.1.
Safety was assessed in a broader group of 105 patients with MCC. The most common side effects (≥10%) included fatigue, muscle and joint pain, itching, diarrhea, rash, fever, and nausea. Serious adverse events occurred in 22% of patients. The recommended dose of Zynyz is 500 mg via IV infusion every four weeks, administered over 30 minutes. Treatment continues until disease progression, unacceptable toxicity, or up to 24 months.

You can read about Merkel Cell Carcinoma (MCC) in Adults: What patients should know on OncoDaily.
The last FDA approval of Zynyz (retifanlimab-dlwr), granted on May 15, 2025, was based on results from the POD1UM-303/InterAACT 2 and POD1UM-202 trials—two key studies evaluating its role in advanced squamous cell carcinoma of the anal canal (SCAC).
In the POD1UM-303/InterAACT 2 Phase 3 trial, Zynyz was tested in combination with carboplatin and paclitaxel as a first-line treatment for patients with inoperable, locally recurrent, or metastatic SCAC. The study enrolled 308 patients and demonstrated that the addition of Zynyz improved progression-free survival (PFS) to 9.3 months versus 7.4 months with chemotherapy alone (HR: 0.63; p = 0.0006). The objective response rate (ORR) was also higher at 56% with Zynyz, compared to 44% in the placebo arm.
In the POD1UM-202 Phase 2 trial, Zynyz was evaluated as monotherapy in 94 patients with advanced SCAC who had progressed on or could not tolerate platinum-based chemotherapy. The study reported an ORR of 14%, with a median duration of response of 9.5 months, supporting its use in the post-platinum setting.

Read more about the FDA approval of retifanlimab-dlwr in combination with chemotherapy and as monotherapy for advanced squamous cell carcinoma of the anal canal on OncoDaily.
Retifanlimab-dlwr side effects and its management
Like other immunotherapies, it can activate the immune system not only against cancer cells but also against healthy tissues, leading to a wide range of side effects. Understanding these adverse events—how often they occur, how they present, and how they are managed—is essential for safe and effective treatment.
Common Side Effects
Most patients experience mild to moderate side effects that are manageable with supportive care. The most commonly reported symptoms include fatigue, muscle and joint pain, pruritus (itching), diarrhea, skin rash, low-grade fever, and nausea. These side effects generally occur in more than 10% of patients. When Zynyz is combined with chemotherapy—such as in the treatment of SCAC—additional effects like peripheral neuropathy, hair loss, and constipation may also arise due to the chemotherapy backbone. These reactions are typically non-life-threatening and can be addressed symptomatically without stopping treatment.
Less Common and Immune-Mediated Side Effects
Less frequently, patients may develop more serious immune-related adverse events. These include inflammation of internal organs such as the lungs (pneumonitis), colon (colitis), liver (hepatitis), kidneys (nephritis), and endocrine glands (thyroiditis, adrenal insufficiency). In rare cases, severe skin reactions such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), or drug reaction with eosinophilia and systemic symptoms (DRESS) may occur. Bloodwork abnormalities, like elevated liver enzymes or decreased lymphocyte counts, can also be seen and may signal the onset of serious toxicity.
Management of Side Effects
The approach to managing side effects from retifanlimab depends on their type and severity. For most mild to moderate reactions, treatment can usually continue with close monitoring and supportive care. However, for more serious immune-related events—typically graded as Grade 3 or 4—therapy should be paused, and high-dose corticosteroids should be initiated to control inflammation. Once symptoms improve to Grade 1 or less, steroids are gradually tapered and treatment may resume if clinically appropriate. In the case of life-threatening toxicities such as severe pneumonitis, fulminant hepatitis, or serious dermatologic syndromes, permanent discontinuation of Zynyz is warranted.
Endocrine-related side effects such as hypothyroidism or adrenal insufficiency may require lifelong hormone replacement therapy. These conditions typically do not necessitate discontinuing Zynyz, as long as the patient is stable on appropriate treatment. Infusion-related reactions may be managed by slowing or halting the infusion, administering antihistamines or steroids, or, if reactions are severe, discontinuing the drug altogether.
Careful monitoring of liver enzymes, kidney function, thyroid hormones, and overall clinical status is crucial throughout treatment to detect emerging toxicities early. With timely recognition and intervention, most side effects of retifanlimab can be managed effectively, allowing patients to continue benefiting from therapy.

What is the Recommended Dosage of Retifanlimab-dlwr?
Zynyz is given as a 500 mg intravenous infusion every four weeks. For Merkel cell carcinoma and for anal cancer patients who have progressed on platinum-based chemotherapy, it is used alone and may be continued for up to 24 months or until disease progression or unacceptable toxicity. For newly diagnosed, inoperable or metastatic anal cancer, Zynyz is combined with carboplatin and paclitaxel for six cycles. After that, treatment may continue with Zynyz alone for up to 12 months.
Dose adjustments are not required for mild kidney or liver impairment, but immune-related side effects—such as pneumonitis, colitis, hepatitis, or severe rash—may require holding treatment and starting steroids. If symptoms don’t improve within 12 weeks or steroids can’t be tapered, the drug should be discontinued. Life-threatening complications or severe infusion reactions also call for permanent discontinuation.

Learn more about Anal Cancer: Symptoms, Causes, Stages, Diagnosis and Treatment on OncoDaily.
How is Retifanlimab-dlwr administered?
Zynyz is given by intravenous infusion and should never be mixed with other drugs in the same IV line. It can be diluted with either 0.9% sodium chloride or dextrose 5% in water (D5W) to a concentration between 1.4 and 10 mg/mL.
Before use, the vial should be checked carefully—its contents must be clear to slightly cloudy, colorless to pale yellow, and free of particles. If the solution is discolored or cloudy, it must be discarded. Do not shake the vial; mix diluted solution gently by inversion. Use PVC, polyolefin, or ethylene vinyl acetate infusion bags for dilution. The infusion should be administered over 30 minutes through a compatible IV line with a suitable in-line filter. Avoid polyurethane infusion sets and never give the drug as a rapid IV push or bolus.
Unopened vials must be refrigerated at 2–8ºC and protected from light. The diluted solution can be kept at room temperature for up to 8 hours or refrigerated for up to 24 hours before use but should be brought to room temperature before infusion. Once removed from refrigeration, it should be infused within 4 hours. Shaking and freezing of both the vial and diluted solution are not recommended.
What to Avoid During Retifanlimab-dlwr Treatment?
During treatment with retifanlimab-dlwr (Zynyz), patients should be vigilant about potential interactions and adverse effects. It’s crucial to avoid coadministering other medications through the same infusion line to prevent possible drug interactions. Additionally, patients should refrain from consuming grapefruit or grapefruit juice, as these can interfere with the metabolism of retifanlimab-dlwr, potentially altering its effectiveness.
Patients are advised to avoid exposure to sunlight or tanning beds without protection, as retifanlimab-dlwr can increase sensitivity to UV rays, heightening the risk of sunburn. It’s also recommended to avoid live vaccines during treatment, as the immune-modulating effects of retifanlimab-dlwr may compromise the body’s ability to respond to these vaccines effectively.
How Long Does the Drug Stay in the Body?
This medication has a long half-life of about 19 days, meaning it stays in the body for an extended period after each dose. Because of this, it doesn’t need to be given frequently and can maintain its effect over time with spaced-out treatments.
Retifanlimab-dlwr effectiveness over time
The effectiveness of retifanlimab-dlwr over time was demonstrated in the final results of the POD1UM-201 trial, a Phase 2 study of patients with advanced Merkel cell carcinoma (MCC), published in the Journal of Clinical Oncology in 2025. In this study of 101 patients, the overall response rate (ORR) was 55%, including 18% who achieved a complete response and 37% with partial responses. The disease control rate was 60%, reflecting patients who experienced tumor shrinkage or stable disease.
Notably, the median duration of response was not reached, with responses lasting beyond 23 months in some cases. Median progression-free survival was 16 months, and median overall survival had not yet been reached, with some patients living beyond 45 months. The safety profile was consistent with expectations for PD-1 inhibitors, with immune-related side effects such as skin reactions and hypothyroidism occurring in a minority of patients. Grade 3 or higher immune-related adverse events occurred in 11% of patients, leading to treatment discontinuation in 9%. These findings underscore the durable and effective activity of retifanlimab in advanced MCC, providing long-lasting tumor control and meaningful survival benefits.
Ongoing trials with Retifanlimab-dlwr
The PERELI study is a Phase 2, open-label trial investigating the combination of pemigatinib, an FGFR inhibitor, and retifanlimab, a PD-1 immune checkpoint inhibitor, in patients with advanced dedifferentiated liposarcoma (DDLPS). DDLPS is an aggressive soft tissue cancer with limited treatment options. Previous research has shown some benefit of immunotherapy alone in this disease, and early studies suggest FGFR inhibitors can slow tumor growth and may enhance the effectiveness of immunotherapy. This study aims to see if combining these two drugs can better control tumor progression in patients who have not responded to first-line treatments.
The RiLEY study is a Phase 1b trial evaluating the combination of 9-ING-41 (elraglusib), a GSK-3β inhibitor, with retifanlimab, a PD-1 immune checkpoint inhibitor, plus modified FOLFIRINOX chemotherapy for patients with advanced pancreatic adenocarcinoma who have not received prior systemic therapy. The study begins with a small safety lead-in cohort to assess tolerability, followed by an expansion phase once a safe dose is established.
This combination is based on the idea that inhibiting GSK-3β may boost immune responses when combined with PD-1 blockade. Both 9-ING-41 and retifanlimab have shown promising safety and anti-tumor activity separately, but their combined effect with chemotherapy is still unknown. The trial aims to explore whether this triple combination can provide enhanced anti-cancer benefits while monitoring for any new or increased side effects.
FAQ
What is Zynyz?
Zynyz (retifanlimab-dlwr) is a humanized IgG4 monoclonal antibody targeting PD-1. By blocking PD-1 interaction with PD-L1 and PD-L2, it restores T-cell function and promotes anti-tumor immunity.
Can Zynyz be used in other cancers not yet FDA approved?
Zynyz is still being studied in clinical trials for several other cancers, such as pancreatic adenocarcinoma and soft tissue sarcomas. Patients with these cancers may access the drug through participation in clinical trials but not as part of standard care unless approved in the future.
Can I receive vaccines while taking Zynyz?
Live vaccines should be avoided during treatment with Zynyz, as they can pose a risk to people with altered immune responses.
Will Zynyz cause hair loss?
Zynyz alone does not typically cause hair loss, but if it’s used in combination with chemotherapy (like in anal cancer), hair loss may occur as a side effect of the chemotherapy drugs, not the immunotherapy.
What are the most common side effects of Zynyz?
The most frequently reported side effects include fatigue, rash, diarrhea, pruritus, arthralgia, myalgia, and fever. These are generally mild to moderate and manageable with supportive care.
What is the half-life of Zynyz?
Retifanlimab-dlwr has a terminal half-life of approximately 19 days, allowing for once-every-4-weeks dosing.
Can Zynyz cause serious side effects?
Yes. Zynyz, like other PD-1 inhibitors, can cause immune-related adverse events, including pneumonitis, colitis, hepatitis, nephritis, endocrinopathies (e.g., hypothyroidism, adrenal insufficiency), and severe dermatologic reactions. High-grade toxicities may require corticosteroids and treatment discontinuation.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
