On May 15, 2025, the U.S. Food and Drug Administration (FDA) approved retifanlimab-dlwr (Zynyz, Incyte Corporation) in combination with carboplatin and paclitaxel for the first-line treatment of adults with inoperable locally recurrent or metastatic squamous cell carcinoma of the anal canal (SCAC). Additionally, the FDA granted approval of retifanlimab-dlwr as monotherapy for adults with locally recurrent or metastatic SCAC with disease progression on or intolerance to platinum-based chemotherapy.
What Is the Rationale for FDA Approval of Retifanlimab-dlwr in SCAC?
Clinical evidence supporting the FDA approval of retifanlimab-dlwr (Zynyz) came from two key trials conducted in different treatment settings for advanced squamous cell carcinoma of the anal canal (SCAC). In the first-line setting, the combination of retifanlimab with chemotherapy was evaluated in the Phase 3 POD1UM-303/InterAACT 2 trial. For patients whose disease had progressed on or who were intolerant to platinum-based chemotherapy, retifanlimab was assessed as a standalone treatment in the Phase 2 POD1UM-202 trial. Each of these studies will be discussed in detail below.
POD1UM-303/InterAACT 2: First-Line Setting
This randomized, double-blind, multicenter Phase 3 trial enrolled 308 patients with inoperable locally recurrent or metastatic SCAC who had not received prior systemic chemotherapy.
Patients in the POD1UM-303/InterAACT 2 trial received standard chemotherapy consisting of carboplatin (AUC 5 on Day 1) and paclitaxel (80 mg/m² on Days 1, 8, and 15) every 28 days for six cycles. Alongside chemotherapy, patients were randomized 1:1 to receive either retifanlimab-dlwr 500 mg intravenously every four weeks or a matching placebo.
Primary Endpoint
The main goal of the trial was to assess progression-free survival (PFS), which was evaluated by a blinded independent central review using RECIST v1.1 criteria. This measurement focused on the length of time patients lived without their cancer worsening.
Key Secondary Endpoints
Additional important outcomes included overall survival (OS), which tracks the length of time patients remain alive after treatment; overall response rate (ORR), which measures the proportion of patients who experienced tumor shrinkage; and duration of response (DOR), indicating how long those responses lasted.
Results
The trial showed that adding retifanlimab-dlwr to chemotherapy improved several important measures of treatment efficacy.
- Median PFS: 9.3 months (retifanlimab) vs 7.4 months (placebo)
- HR: 0.63 (95% CI: 0.47–0.84), p = 0.0006
- Interim OS: 29.2 months vs 23.0 months
- HR: 0.70 (95% CI: 0.49–1.01) (not statistically significant)
- ORR: 56% (retifanlimab) vs 44% (placebo)
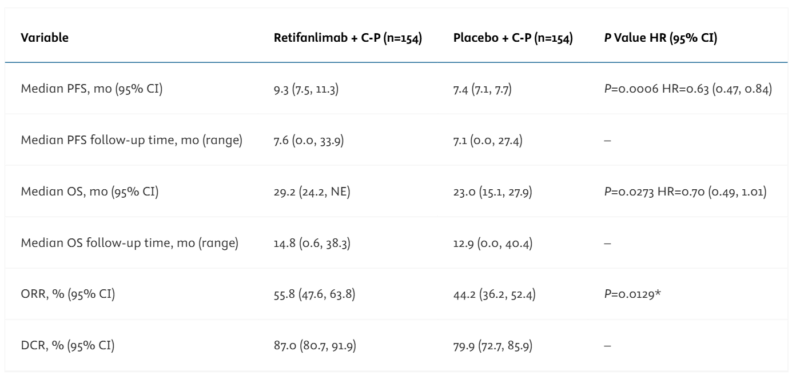
The pivotal findings from the POD1UM-303/InterAACT 2 trial were published in ESMO Annals of Oncology in September 2024.
POD1UM-202: Previously Treated SCAC
This was an open-label, multicenter, single-arm Phase 2 trial in 94 patients with previously treated SCAC (post-platinum failure or intolerance). Patients received retifanlimab-dlwr 500 mg IV every 4 weeks until progression or unacceptable toxicity, for up to 24 months.
Results
- ORR: 14% (95% CI: 8–23)
- Median DOR: 9.5 months (95% CI: 4.4–NE)
Dosing and Safety Information
The recommended dosing for this drug varies depending on the treatment approach. When combined with chemotherapy, it is administered at 500 mg every four weeks for up to 12 months. As a monotherapy, the dose remains 500 mg every four weeks but can be continued for up to 24 months. Patients should be aware of important safety warnings, which include the risk of immune-mediated adverse reactions, infusion-related reactions, complications following allogeneic hematopoietic stem cell transplantation (HSCT), and potential embryo-fetal toxicity.
You can find more information about retifanlimab-dlwr approval on the FDA official website.
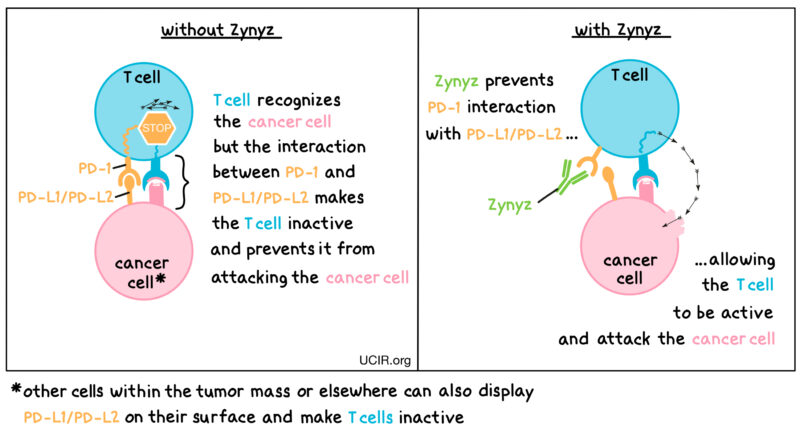
How Retifanlimab Works?
Retifanlimab-dlwr is a humanized IgG4 monoclonal antibody that targets the programmed death-1 (PD-1) receptor found on T cells—a key component of the body’s immune system. Under normal circumstances, when PD-1 binds to its ligands PD-L1 or PD-L2, it sends an “off” signal that dampens T-cell activity. This mechanism is meant to prevent overactivation of the immune system, which could harm normal tissues. However, many cancers, including squamous cell carcinoma of the anal canal (SCAC), take advantage of this pathway to evade immune detection and destruction.
By blocking the interaction between PD-1 and its ligands, retifanlimab removes this immune “brake”, allowing T cells to remain active. This helps the immune system recognize, attack, and kill cancer cells more effectively. This mechanism of action is particularly relevant in SCAC, a cancer often associated with chronic viral infection (such as HPV), which may increase immune system engagement but also lead to immune exhaustion. Retifanlimab aims to reinvigorate exhausted T cells and restore a durable anti-tumor response.
Anal Cancer Overview
Anal cancer is a rare but increasingly diagnosed cancer, often linked to HPV infection. It can cause symptoms like bleeding, itching, pain, and lumps near the anus, which are sometimes mistaken for hemorrhoids—leading to delayed diagnosis.
HPV infection, smoking, weakened immunity (like in HIV), and certain sexual behaviors are major risk factors. Most anal cancers are squamous cell carcinomas and respond well to combined chemotherapy and radiation, especially when caught early.
Diagnosis includes a rectal exam, biopsy, and imaging like CT, MRI, or PET scans. In high-risk or unclear cases, genetic testing may guide treatment. Anal cancer is staged from I (localized small tumor) to IV (spread to distant organs).
Treatment typically involves chemotherapy and radiation. Surgery is considered when other therapies fail. Early-stage tumors may be removed surgically, while advanced cases might require extensive operations like abdominoperineal resection. Common chemotherapy options include 5-FU/mitomycin or capecitabine-based regimens. In metastatic disease, carboplatin and paclitaxel are preferred. Immunotherapy and newer combinations are under investigation.
The FDA’s approval of Retifanlimab-dlwr (Zynyz) marks a significant advance in the treatment of advanced anal cancer. Data from POD1UM-303 and POD1UM-202 support its use as a first-line option in combination with chemotherapy and as a monotherapy for previously treated patients. This dual approval underscores the growing role of immunotherapy in the management of HPV-related cancers.

Learn more about Anal Cancer: Symptoms, Causes, Stages, Diagnosis and Treatment on OncoDaily.


