Cardio-Oncology in Focus: How Chemotherapy Can Harm Heart Health
The field of cardio-oncology has emerged as a critical intersection between cardiology and oncology, driven by the recognition that life-saving cancer therapies can lead to significant cardiovascular complications. Advances in cancer treatments, including chemotherapy, targeted therapies, and immunotherapies, have dramatically improved survival rates but have also introduced risks such as heart failure, arrhythmias, and vascular dysfunction. These complications can arise from both traditional and modern therapies, underscoring the need for specialized care to manage these dual health challenges.
Photo: Depositphotos
This article explores the vital role of Cardio-Oncology in addressing chemotherapy-induced cardiotoxicity, highlighting key risk factors, early detection strategies, and current approaches to prevention and management.
What Role Does Cardio-Oncology Play in Modern Cancer Care?
Cardio-oncology focuses on preventing, identifying, and managing cardiovascular toxicities associated with cancer treatments. This multidisciplinary field aims to balance oncologic efficacy with cardiac safety through early risk stratification, continuous monitoring using advanced imaging techniques and biomarkers, and the development of tailored treatment strategies. Recent research highlights the importance of structured cardio-oncology programs that integrate cardiologists and oncologists to provide coordinated care throughout the cancer treatment process.
This section sets the stage for the emerging field of cardio-oncology, highlighting the growing recognition that life-saving cancer treatments can carry significant cardiovascular risks. It briefly outlines the importance of balancing oncologic efficacy with cardiac safety in patient care.
What Is the Mechanism Behind Chemotherapy- Induced Cardiotoxicity?
Chemotherapy-induced cardiotoxicity involves complex cellular and molecular mechanisms that impair cardiac function, primarily through oxidative stress, mitochondrial dysfunction, and direct cardiomyocyte injury.
Oxidative Stress and Reactive Oxygen Species (ROS)
Chemotherapeutic agents like anthracyclines (e.g., doxorubicin) and platinum-based drugs (e.g., cisplatin) generate excessive ROS through redox cycling, disrupting cellular homeostasis. For example:
- Anthracyclines form iron complexes that catalyze free radical production, overwhelming endogenous antioxidant defenses.
- ROS induce lipid peroxidation, protein oxidation, and DNA damage in cardiomyocytes, leading to apoptosis and necrosis.
- Persistent oxidative stress triggers inflammatory pathways (e.g., NF-κB) and exacerbates cardiac remodeling.
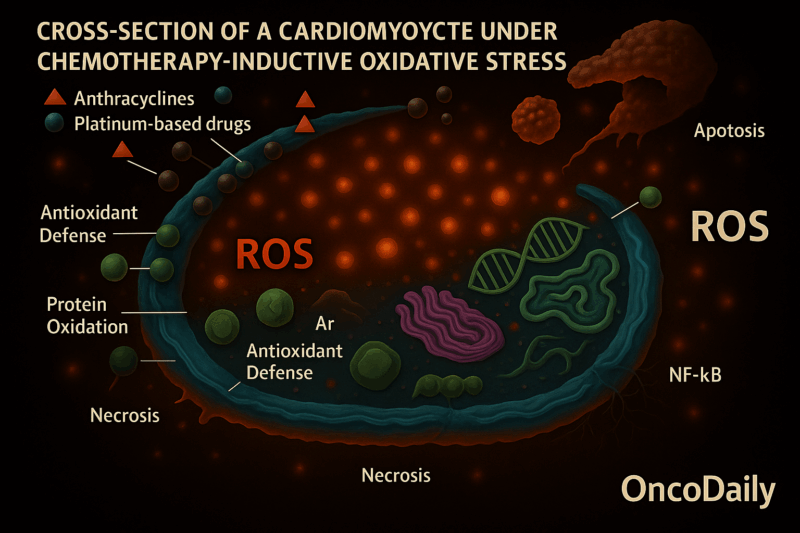
Anthracyclines form Iron Complexes that Catalyze Free Radical Production, Overwhelming Endogenous Antioxidant Defenses.
Anthracyclines like doxorubicin can bind to iron and form complexes. These iron–anthracycline complexes participate in chemical reactions known as Fenton reactions, which produce free radicals—particularly reactive oxygen species (ROS). Under normal conditions, the body relies on antioxidant systems such as glutathione, catalase, and superoxide dismutase to neutralize ROS and maintain cellular balance. However, the excessive ROS generated by these iron-anthracycline complexes overwhelm the body’s natural antioxidant defenses, leading to oxidative stress and cellular damage.
ROS Induce Lipid Peroxidation, Protein Oxidation, and DNA Damage in Cardiomyocytes, Leading to Apoptosis and Necrosis.
The reactive oxygen species (ROS) attack various cellular components. Lipid peroxidation damages cell membranes, making them leaky or dysfunctional. Protein oxidation alters the structure and function of key enzymes and proteins. DNA damage results in errors within the genetic material, which the cells may not be able to repair. These types of damage lead to the death of heart muscle cells, or cardiomyocytes, through two main pathways: apoptosis, which is a form of programmed cell death, and necrosis, which is an uncontrolled and often damaging form of cell death.
Persistent Oxidative Stress Triggers Inflammatory Pathways (e.g., NF-κB) and Exacerbates Cardiac Remodeling.
When ROS production is ongoing, it doesn’t just damage cells—it also activates inflammatory signaling pathways such as NF-κB. Activation of NF-κB leads to the production of inflammatory cytokines and increases cellular stress. This chronic inflammation and sustained injury trigger structural and functional changes in the heart, a process known as cardiac remodeling. The remodeling may involve thickening or thinning of the heart walls and the development of fibrosis, all of which progressively impair cardiac function and can eventually lead to heart failure.
Mitochondrial Dysfunction
Mitochondria are critical targets of chemotherapy due to their role in energy production and signaling:
- mtDNA damage: Chemotherapy agents cause mitochondrial DNA deletions and mutations, impairing oxidative phosphorylation and ATP synthesis.
- Mitochondrial membrane instability: ROS destabilize the mitochondrial membrane potential, promoting mitochondrial permeability transition pore (mPTP) opening, which releases pro-apoptotic factors (e.g., cytochrome c).
- Calcium dysregulation: Altered mitochondrial Ca²⁺ handling disrupts contractility and increases arrhythmia risk.
- Emerging pathways: Mitochondria also influence epigenetic regulation and innate immunity, contributing to long-term cardiovascular complications.
Direct Cardiomyocyte Injury
Chemotherapy directly damages cardiomyocytes through:
- DNA damage response: Anthracyclines inhibit topoisomerase IIβ, causing double-strand DNA breaks and activating TP53-mediated apoptosis.
- Transcriptomic changes: Upregulation of pro-inflammatory genes (e.g., JUN, NF-κB) and fibrosis-related pathways (e.g., TGF-β) leads to contractile dysfunction and cardiac remodeling.
- Post-translational modifications (PTMs): Ubiquitination and phosphorylation alterations disrupt protein function, exacerbating mitochondrial and metabolic dysfunction.
Clinical Impact of Cardiotoxicity
A 2024 meta-analysis of 53 studies (35,651 patients) found: The incidence of chemotherapy-related cardiac dysfunction is 63.21 per 1,000 person-years, peaking within 6 months post-treatment. Older patients (≥50 years) and those with breast cancer, leukemia, or lymphoma face higher risks. Subclinical mitochondrial damage often progresses to symptomatic heart failure if untreated. Hai-Wei Deng , Rui Fan, PubMed Central , 2024
Mitigation Strategies
Recent advances focus on mitochondria-targeted antioxidants (MTAs) like elamipretide and melatonin, which reduce ROS and stabilize mitochondrial function. Additionally, ACE inhibitors and beta-blockers are used clinically, though they primarily manage symptoms rather than prevent cardiomyocyte death.
In summary, chemotherapy-induced cardiotoxicity arises from interconnected pathways involving oxidative stress, mitochondrial damage, and direct cardiomyocyte injury. Emerging therapies targeting mitochondrial signaling and PTMs offer promising avenues to protect cardiac function in cancer patients. Yan Ma , J Xenobiot , 2025 , Jean C. Bikomeye , American Journal of Physiology, 2022
What Are The Most Common Cardiotoxic Chemotherapeutic Agents ?
Chemotherapy-induced cardiotoxicity manifests through distinct mechanisms depending on the drug class. Anthracyclines like doxorubicin and epirubicin generate reactive oxygen species (ROS) that cause oxidative stress and cardiomyocyte damage, leading to dose-dependent heart failure and left ventricular dysfunction. Fluoropyrimidines (5-fluorouracil, capecitabine) induce coronary vasospasm via nitric oxide depletion, resulting in chest pain, arrhythmias, and myocardial infarction. Taxanes such as paclitaxel and docetaxel directly affect the cardiac conduction system, causing bradycardia, tachyarrhythmias, and myocardial ischemia; they also exacerbate anthracycline toxicity by stimulating formation of the cardiotoxic metabolite doxorubicinol.
Alkylating agents like cyclophosphamide trigger oxidative stress and endothelial damage, leading to hemorrhagic myocarditis, arrhythmias, and heart failure through metabolites like acrolein. Platinum agents (e.g., cisplatin) generate ROS and cause electrolyte disturbances, resulting in arrhythmias, chest pain, and myocardial infarction, with chronic exposure causing vascular endothelial dysfunction even before systemic cardiovascular changes. These cardiotoxic effects necessitate careful risk stratification and monitoring during cancer treatment.
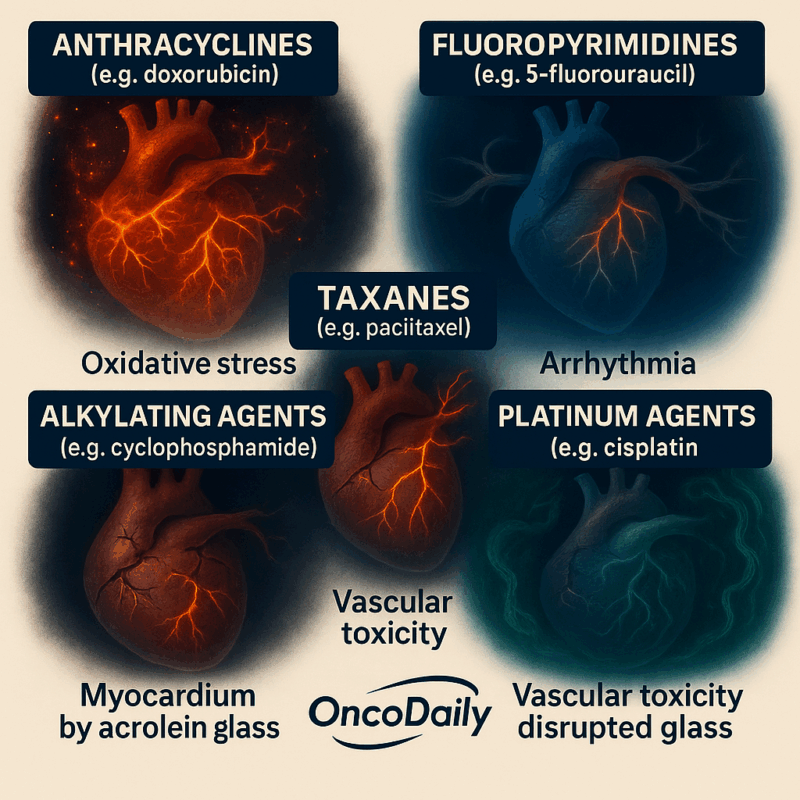
Cardiotoxicity from these agents can be acute, subacute, or chronic, and may occur at any time from initiation of therapy to years after completion. Mechanisms include oxidative stress, mitochondrial dysfunction, electrolyte imbalance.. Muhammad T. Amjad; Anusha Chidharla; Anup Kas , National Library of Medicine , 2023
What Are the Types of Cardiovascular Complications?
Heart Failure (HF)
HF is a primary manifestation of cardiotoxic effects from cancer therapies, particularly anthracyclines and trastuzumab. These drugs can lead to dilated cardiomyopathy and reduced left ventricular function. HF risk is dose-dependent and can occur both during and after treatment. Early detection through biomarkers and imaging is crucial for managing HF effectively.
Arrhythmias
Arrhythmias are associated with various cancer therapies, including anthracyclines and immune checkpoint inhibitors (ICIs). They can also occur during cytokine release syndrome (CRS) in CAR-T cell therapy. Arrhythmias can occur acutely during treatment, especially with CRS, and may require immediate intervention.
Hypertension
Tyrosine kinase inhibitors (TKIs), such as VEGF inhibitors, are linked to hypertension due to their effects on endothelial function and vascular resistance. Hypertension can develop during treatment and may necessitate aggressive blood pressure management to prevent cardiovascular events.
Myocarditis
Myocarditis is a recognized side effect of ICIs and can occur within the first three months of treatment. Early recognition and management are critical, as myocarditis can lead to severe cardiac dysfunction if untreated.
Pericardial Disease
Pericardial disease, including pericarditis and effusion, is associated with certain TKIs and ICIs.
Pericardial complications can arise during or shortly after treatment and require careful monitoring.
Ischemia
Ischemia can result from therapies that affect vascular function, such as VEGF inhibitors, leading to increased vascular resistance and potential coronary events. Ischemic events may occur during treatment due to compromised endothelial function and heightened vascular resistance.
Variations in Timing and Severity
Different cancer therapies have distinct cardiotoxic profiles. For example, anthracyclines primarily cause HF, while TKIs may lead to hypertension and ischemia. Patient-specific factors, such as pre-existing heart conditions and genetic predispositions, influence the risk and severity of cardiac complications.
Cardiac complications can occur during treatment (e.g., arrhythmias with CRS) or years after treatment (e.g., late-onset HF from anthracyclines).
In summary, cardio-oncology emphasizes the importance of early detection and management of cardiac complications associated with cancer therapies to mitigate adverse outcomes and improve patient survival and quality of life. Detail the spectrum of cardiac conditions that may arise, including heart failure, arrhythmias, hypertension, myocarditis, pericardial disease, and ischemia. Emphasize how these complications may vary in timing and severity.
What Are the Risk Factors of Cardiotoxicity?
Risk factors for cardiotoxicity include both modifiable and non-modifiable elements that influence susceptibility to cardiac damage, particularly in the context of cancer therapies such as anthracyclines.
Non-Modifiable Risk Factors
Older age is a significant non-modifiable risk factor, with increased risk notably in patients over 50 years for trastuzumab and over 65 years for anthracyclines.
History of cardiovascular disorders, such as heart failure, coronary artery disease, or cardiomyopathies, increases vulnerability to cardiotoxicity. Family history of premature cardiovascular disease or genetic factors can heighten risk. Female gender and certain ethnic backgrounds may also influence risk, though these are less emphasized in recent studies.
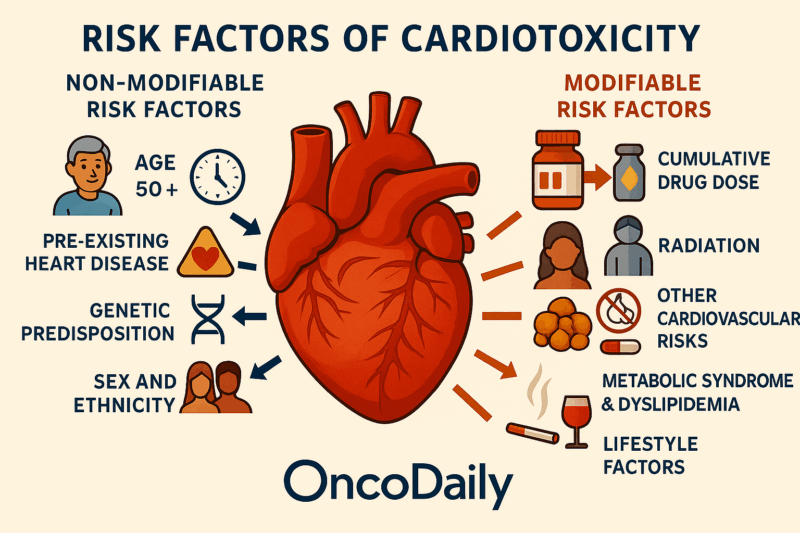
Modifiable Risk Factors
Higher cumulative doses of cardiotoxic agents like anthracyclines are strongly linked to increased cardiotoxicity risk.
Radiation Exposure: Adjunctive radiotherapy, especially to the chest or mediastinum, potentiates cardiotoxic effects. A major modifiable factor that significantly increases risk for coronary artery disease, heart failure, valvular disease, and arrhythmias, often amplifying the effects of cardiotoxic therapies.
Diabetes mellitus, hyperlipidemia, obesity, tobacco use, and sedentary lifestyle contribute to increased cardiotoxicity risk. These conditions further augment cardiovascular risk in survivors exposed to cardiotoxic treatments. Smoking, high alcohol intake, and physical inactivity are modifiable behaviors that increase susceptibility.
Patients undergoing cardiotoxic cancer therapies should be assessed for both modifiable and non-modifiable risk factors. Non-modifiable factors like age, genetic predisposition, and pre-existing heart disease define baseline risk, while modifiable factors such as hypertension, cumulative drug dose, radiation exposure, and lifestyle factors offer targets for intervention to reduce cardiotoxicity risk. Optimizing control of modifiable cardiovascular risk factors prior to and during treatment is crucial to mitigate cardiotoxic effects.
This comprehensive risk assessment approach aligns with recent literature emphasizing personalized management to improve cardiac outcomes in patients receiving potentially cardiotoxic therapies. Andre Gabriel , 2019, Jieli Tong, 2025
Discuss both modifiable and non-modifiable factors that increase susceptibility to cardiotoxicity, such as age, pre-existing heart disease, cumulative drug dose, radiation exposure, and genetic predispositions.
Leonardo Da Vinci human heart drawing, between 1507 and 1513
What Are the Strategies for Monitoring and Early Detection of Cardiotoxicity?
Baseline cardiac evaluation is essential before initiating potentially cardiotoxic cancer therapies. This typically includes clinical assessment and baseline imaging to establish cardiac function status.
Echocardiography
Echocardiography, especially transthoracic echocardiography, is the primary imaging modality for monitoring cardiotoxicity, as it allows for the assessment of left ventricular ejection fraction (LVEF) and diastolic function. Advanced echocardiographic techniques, such as global longitudinal strain (GLS) measured by speckle-tracking echocardiography, offer sensitive early detection of subclinical myocardial dysfunction before any changes in LVEF become apparent. Additionally, the Tei index (myocardial performance index), a Doppler echocardiographic parameter of global ventricular function, has shown promise in detecting subclinical myocardial alterations following anthracycline therapy, even when LVEF remains within normal limits.
Cardiac Magnetic Resonance Imaging (MRI)
Cardiac MRI offers detailed structural and functional assessment and tissue characterization, useful for detecting early cardiotoxicity and fibrosis. It is increasingly used as a complementary tool to echocardiography for more precise evaluation, especially when echocardiographic images are suboptimal or when detailed tissue characterization is needed.
Biomarkers in Ongoing Monitoring
Cardiac Troponins (cTnI and cTnT)
Cardiac troponins (cTnI and cTnT) are highly specific markers of myocardial injury, and high-sensitivity assays can detect even minor myocardial damage early during cancer therapy. Elevated troponin levels during or after chemotherapy indicate cardiomyocyte injury and are predictive of subsequent cardiac dysfunction. However, troponin elevations may be low-level and transient, and their interpretation should be integrated with imaging findings. Ultrasensitive detection platforms further enhance the early identification of cardiotoxicity by detecting subtle increases in troponin I.
Natriuretic Peptides (BNP and NT-proBNP)
BNP and NT-proBNP are markers of myocardial wall stress and heart failure, and their levels can rise transiently during chemotherapy due to altered cardiac loading conditions. Measurements of BNP or NT-proBNP have good negative predictive value for heart failure and can assist in early detection and monitoring, although their specificity may be limited by other conditions such as renal failure or hyperthyroidism. While biomarker-guided therapy using BNP or NT-proBNP in established heart failure remains controversial, it is considered useful for early intervention in at-risk patients.
Integrated Approach and Recommendations
Current strategies emphasize a multimodal approach combining serial imaging (echocardiography with GLS, cardiac MRI when needed) and biomarker monitoring (troponin and natriuretic peptides) for early detection of cardiotoxicity. Echocardiographic parameters such as GLS and the Tei index can detect subclinical dysfunction earlier than LVEF decline. Biomarkers provide additional, minimally invasive tools for early myocardial injury detection.
Regular monitoring schedules depend on the type of therapy and patient risk factors, with more frequent assessments for high-risk regimens like anthracyclines and HER2-targeted therapies.
This integrated monitoring strategy enables early identification of cardiotoxicity, allowing timely intervention to prevent progression to symptomatic heart failure. Further research is ongoing to optimize screening protocols and validate novel imaging and biomarker tools. J. Clin. Med. , MDPI , 2024
You Can Also Read Ocular Side Effects of Chemotherapy: Understanding the Risks of Eye Toxicity by OncoDaily

How Can Cardiotoxicity be Effectively Prevented and Managed?
Prevention and management of cancer therapy-related cardiotoxicity rely on risk stratification, dose optimization, early use of cardioprotective medications, lifestyle interventions, and coordinated multidisciplinary care. These strategies reduce cardiac complications, allow continuation of effective cancer therapy, and improve long-term cardiovascular health and quality of life in cancer patients.
Dose Adjustments and Therapy Optimization
Limiting the use and dosage of cardiotoxic agents such as anthracyclines and HER2-targeted therapies is a cornerstone of primary prevention to reduce cardiac risk while maintaining oncologic efficacy. For patients at high risk of cardiotoxicity, alternative, less cardiotoxic regimens may be considered, with individualized therapy plans developed through multidisciplinary collaboration.
Cardioprotective Medications
Pharmacologic prevention with ACE inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, mineralocorticoid receptor antagonists, and statins has shown benefit in reducing the incidence and severity of cardiotoxicity. Early initiation of these agents, especially in high-risk patients or those with early signs of cardiac injury, can preserve left ventricular function and prevent progression to heart failure. Dexrazoxane remains a specific cardioprotective agent used to prevent anthracycline-induced cardiotoxicity. Emerging evidence suggests potential benefits of sodium-glucose cotransporter 2 (SGLT2) inhibitors, though data are preliminary and require further validation.
Lifestyle Interventions and Rehabilitation
Lifestyle modification, including smoking cessation, blood pressure control, weight management, and regular physical activity, is recommended to mitigate cardiovascular risk factors in cancer patients. Cardiac rehabilitation programs, incorporating exercise training and physical therapy, improve cardiovascular fitness and reduce systemic inflammation, thereby potentially lowering the risk of cardiotoxicity. Tele-rehabilitation approaches have also been proposed to enhance accessibility and adherence to these rehabilitation programs.
Dual Expertise, Singular Goal: Protecting the Heart in Cancer Care
Integrated cardio-oncology care involving oncologists, cardiologists, and other specialists is essential for comprehensive risk assessment, monitoring, and management throughout cancer treatment and survivorship. Such collaboration enables timely cardiac evaluation before, during, and after therapy, facilitating early detection and intervention for cardiotoxicity. Multidisciplinary teams help balance oncologic treatment goals with cardiovascular safety, thereby optimizing patient outcomes.
This synthesis is drawn from recent comprehensive reviews and clinical guidelines published between 2022 and 2025, reflecting the latest evidence-based cardio-oncology practices. MDPI Christos Kourkek , National Library of Medicine, 2022
Please remember: All of these treatments should only be used after careful evaluation and recommendation by your cardiologist and oncologist. Do not take risks by diagnosing or treating yourself without professional guidance. Always consult your healthcare team to ensure your safety and the best possible outcome.
How Important Is Ongoing Cardiac Surveillance for Long-Term Heart Health?
Recent evidence from the last five years highlights the critical importance of ongoing cardiac surveillance and long-term cardiovascular disease (CVD) management in cancer survivors, especially those exposed to high-risk treatments such as anthracyclines, platinum-based chemotherapy, hormonal therapies, and thoracic radiotherapy.
Cancer survivors remain at elevated risk for a broad spectrum of cardiovascular diseases, including heart failure, cardiomyopathy, arrhythmias, coronary artery disease, stroke, and valvular heart disease, which may emerge many years after treatment completion. Despite this risk, cardiovascular screening and management are often not integrated into routine long-term cancer care, underscoring the need for systematic surveillance protocols tailored to individual risk profiles. Surveillance should include regular cardiac imaging, biomarker assessments, and clinical evaluations, particularly for survivors exposed to cardiotoxic therapies and younger survivors who may have higher relative risk. Multidisciplinary cardio-oncology care teams are essential to coordinate surveillance and early detection, involving oncologists, cardiologists, primary care providers, and other specialists.

Long-Term Management of Cardiovascular Disease
Long-term management involves personalized care plans focusing on prevention, early detection, and treatment of cardiovascular disease, including pharmacologic therapies such as ACE inhibitors, beta blockers, statins, and aspirin when indicated. Lifestyle interventions—such as smoking cessation, physical activity, healthy diet, and weight management—are critical components to reduce cardiovascular risk and improve survivorship outcomes. Survivorship care plans should address evolving cardiovascular risks and comorbidities over time, emphasizing continuous risk assessment and patient education to empower self-management. Special attention is warranted for specific populations, such as multiple myeloma survivors under 50 years old, who exhibit significantly higher cardiovascular disease risk and require vigilant monitoring and tailored interventions.
Integration of cardio-oncology services into cancer survivorship programs facilitates coordinated care, reduces fragmented management, and improves quality of life for survivors. Ongoing cardiac surveillance and comprehensive long-term cardiovascular care are imperative for cancer survivors exposed to high-risk treatments to mitigate late cardiotoxicity and improve overall survival and quality of life. Multidisciplinary, personalized approaches combining regular monitoring, pharmacologic management, lifestyle modification, and patient-centered education represent the current best practice in survivorship cardiac care. Jaidyn Muhandiramge, 2022
You Can Also Watch ASCO24 Updates: Cancer Therapy & Heart Health: Insights from Experts and Survivors by Oncodaily
Why Is a Multidisciplinary Cardio-Oncology Approach Essential in Cancer Treat
Integrating cardiovascular care into oncology protocols is crucial to improving outcomes for patients facing the dual challenges of cancer and heart health. Recent evidence underscores that early cardiovascular risk management, including GLS-guided cardioprotective therapy and structured exercise programs, significantly reduces the incidence of chemotherapy-related cardiotoxicity and preserves cardiac function, thereby enhancing quality of life and survival rates in cancer patients. Given the shared risk factors and the cardiotoxic potential of many cancer therapies, a multidisciplinary cardio-oncology approach is essential to balance effective cancer treatment with cardiovascular safety.
Increased awareness among healthcare providers and patients about the risks of cardiotoxicity enables early intervention through baseline cardiac assessments, biomarker monitoring, and timely initiation of cardioprotective medications. Moreover, ongoing research into biomarker-based strategies and advanced imaging techniques promises to refine early detection and personalized management of cardiotoxicity. The establishment of standardized protocols for integrating cardiovascular risk management into cancer care pathways remains a priority to optimize patient outcomes.
Therefore, fostering collaboration between oncologists, cardiologists, and allied health professionals, alongside continued research investment, is imperative to address the growing burden of cardiovascular complications in cancer survivors. This integrated approach will ensure that cancer therapies are delivered safely without compromising long-term cardiovascular health, ultimately improving survival and quality of life for this vulnerable population. Segundo Fernando Morales Quilligana, 2024, International Journal of Research in Medical Sciences
Written by Milena Shekoyan MD
FAQ
What is chemotherapy-induced cardiotoxicity?
It refers to heart damage caused directly or indirectly by chemotherapy drugs, ranging from mild myocardial dysfunction to severe heart failure.
Which chemotherapy drugs are most commonly associated with cardiotoxicity?
Anthracyclines (e.g., doxorubicin), trastuzumab, 5-fluorouracil, cyclophosphamide, and taxanes are among the main agents known to cause cardiotoxic effects.
What are the mechanisms behind chemotherapy-induced cardiotoxicity?
Mechanisms include direct myocardial cell toxicity, oxidative stress, mitochondrial dysfunction, inflammation, vascular damage, arrhythmias, and disruption of cardiac signaling pathways.
What are the clinical manifestations of cardiotoxicity from chemotherapy?
Symptoms vary from asymptomatic changes in cardiac biomarkers to arrhythmias, myocardial ischemia, heart failure, and sudden cardiac death.
How soon after chemotherapy can cardiotoxicity appear?
Cardiotoxicity can be acute (during or within 2 weeks after treatment), early chronic (within 1 year), or late chronic (years after therapy completion).
How is chemotherapy-induced cardiotoxicity diagnosed?
Diagnosis involves cardiac imaging (echocardiography, cardiac MRI), measurement of cardiac biomarkers (troponins, natriuretic peptides), and clinical evaluation of symptoms.
Can chemotherapy-induced cardiotoxicity be prevented?
Strategies include careful monitoring, dose adjustment, use of cardioprotective agents, and interdisciplinary cardio-oncology care to balance cancer treatment efficacy and cardiac safety.
Is chemotherapy-induced cardiotoxicity reversible?
It depends on the drug and severity; for example, anthracycline-induced damage is often irreversible, while trastuzumab-related cardiotoxicity may be reversible upon treatment modification.
What is the impact of cardiotoxicity on cancer treatment?
Cardiotoxicity can lead to interruption or modification of chemotherapy, potentially affecting cancer treatment outcomes and long-term survivorship care.
What follow-up care is recommended for patients at risk of cardiotoxicity?
Long-term cardiac monitoring and management by a multidisciplinary team are essential, especially for breast cancer survivors receiving cardiotoxic chemotherapy agents
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
