Bishal Gyawali/X
Jun 6, 2025, 15:47
CHALLENGE Trial at ASCO 2025: Evaluating the Evidence and Implementation of Exercise in Cancer Care
Nina Niu Sanford, Assistant Professor and Chief of Gastrointestinal Radiation Oncology at Harvard/Brigham and Women’s Hospital/Massachusetts General Hospital, shared a post on X by John Mandrola, Editor of Sensible Medicine, adding:
“These are such great points regarding: CHALLENGE and I agree with most, but:
1) I actually do think the effect size is believable…the absolute gain from a lot of the cancer drugs isn’t that much. And of course when you put it as a %, the difference seems much more impressive than absolute numbers.
2) Would the cost of implementing such a program really be massive? Especially in comparison to currently approved therapies/drugs? A lot of the program could probably be done by AI in 2025.
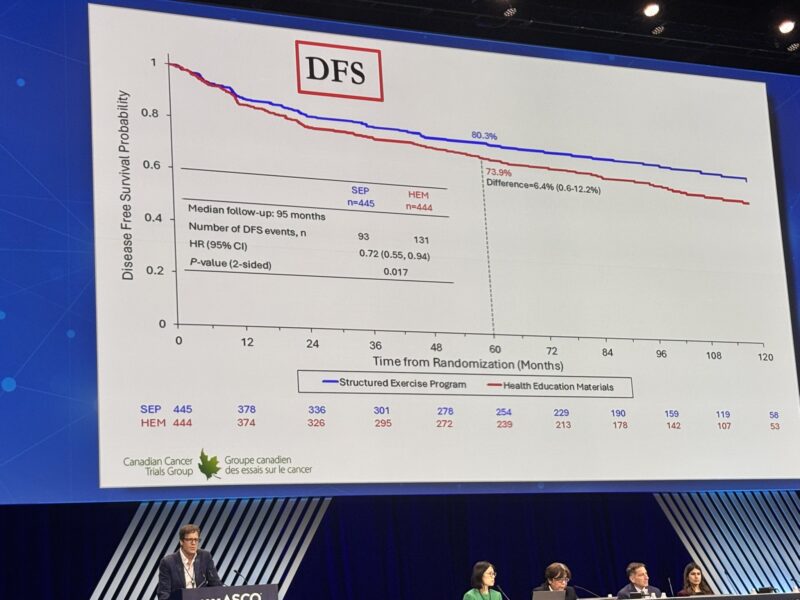
3) The intervention group definitely received more attention during the 3 years, but the curves continue to separate and results presented were for 8 years. So it’s different than say tumor treating fields in pancreas cancer (also an ASCO 2025 presentation) where patients wore the device until death. There, more frequent healthcare interactions could definitely have contributed to better survival.
4) I’m not as familiar with regulatory policy – but it seems drugs, devices (see histotripsy)…protons have pathways for approval based on less robust data. Do they always require confirmatory Phase 3 RCTs?
Timothée Olivier made some great points re: censoring.
Since Aaron Goodman was planning to interview Chris Booth, maybe he can ask him to comment on all these points.”
Quoting John Mandrola‘s post:
“Good morning all. After yesterday’s excitement about the CHALLENGE exercise trial, here is my critical appraisal on Sensible Medicine.”
Later Nina Niu Sanford shared another post by Bishal Gyawali, Associate Professor at Queen’s University on X, adding:
“Just brand it “Exercizumab!”
In all seriousness, I have been recommending exercise during and after treatment to all my patients who are able to.
Great to have Level 1 evidence supporting this strategy.
Now need systematic change to upscale and implement.”
Quoting Bishal Gyawali‘s post:
“Such a well deserved standing ovation for Chris Booth and the Challenge trial.
These data are quite powerful. Much stronger evidence than what we saw in plenaries today.”
Mark Lewis, Director of Gastrointestinal Oncology at Intermountain Healthcare in Utah, shared a post on X, reffering Nina Niu Sanford‘s post:
“Exercizumab?!
Hmm, on second thought might be addictive and have consequences of GAINS.”
Read more thoughts on CHALLENGE Trial on OncoDaily.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
Aug 25, 2025, 09:45
Aug 25, 2025, 09:09
Aug 24, 2025, 21:47
Aug 24, 2025, 21:22