
JAVELIN Bladder Medley Results: Avelumab + SG vs Avelumab in 1L Maintenance of Advanced UC
The phase 3 JAVELIN Bladder 100 trial demonstrated that avelumab first-line (1L) maintenance combined with best supportive care (BSC) significantly prolonged overall survival (OS) and progression-free survival (PFS) compared to BSC alone in patients with advanced urothelial carcinoma (aUC) without progression following platinum-based chemotherapy (PBC). The ongoing phase 2 JAVELIN Bladder Medley trial investigates whether combining avelumab with other antitumor agents, such as sacituzumab govitecan (SG)—a Trop-2–directed antibody-drug conjugate—improves efficacy and safety compared to avelumab monotherapy in this patient population.
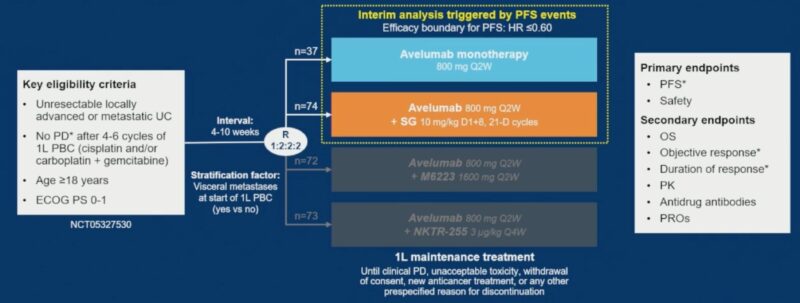
Methods
Eligible patients had unresectable locally advanced or metastatic urothelial carcinoma, Eastern Cooperative Oncology Group (ECOG) performance status 0–1, and no disease progression after 4–6 cycles of 1L PBC. Patients were randomized 2:1 to receive either avelumab plus SG or avelumab monotherapy. Randomization was stratified by the presence of visceral metastases at the start of 1L PBC. The primary endpoints were investigator-assessed progression-free survival (PFS) and safety; overall survival (OS) was a secondary endpoint. For PFS and OS analyses, data from the avelumab monotherapy arm were extended per protocol using propensity score-weighted data from the JAVELIN Bladder 100 trial.
Results of JAVELIN Bladder Medley Results
At data cutoff (September 16, 2024), 38 of 74 patients (51.4%) in the avelumab plus SG arm and 10 of 37 patients (27.0%) in the avelumab monotherapy arm were still receiving study treatment. Baseline characteristics were balanced between arms, with median ages of 70 and 67 years, respectively. Visceral metastases were present in 50.0% (combination) and 51.4% (monotherapy) of patients, and a lower proportion of patients in the combination arm had ECOG performance status of 1 (31.1% vs 54.1%).
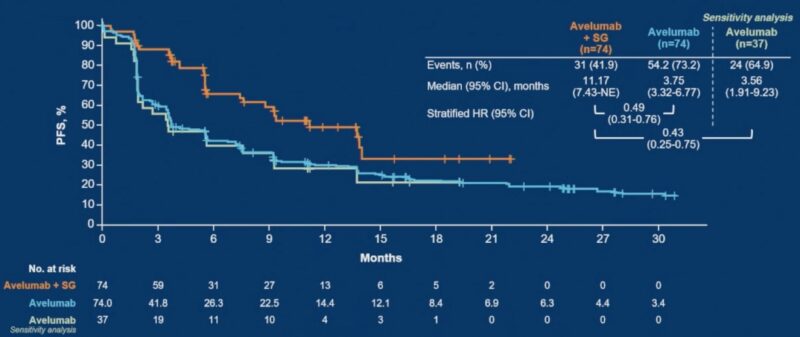
- Progression-Free Survival: Median PFS was 11.17 months (95% CI, 7.43–not estimable) in the avelumab plus SG arm versus 3.75 months (95% CI, 3.32–6.77) in the avelumab monotherapy arm (hazard ratio [HR], 0.49; 95% CI, 0.31–0.76).
- Overall Survival: Data were immature at cutoff; median OS was not reached (95% CI, 15.51–not estimable) in the combination arm versus 23.75 months (95% CI, 18.79–30.82) in the monotherapy arm (HR, 0.79; 95% CI, 0.42–1.50).
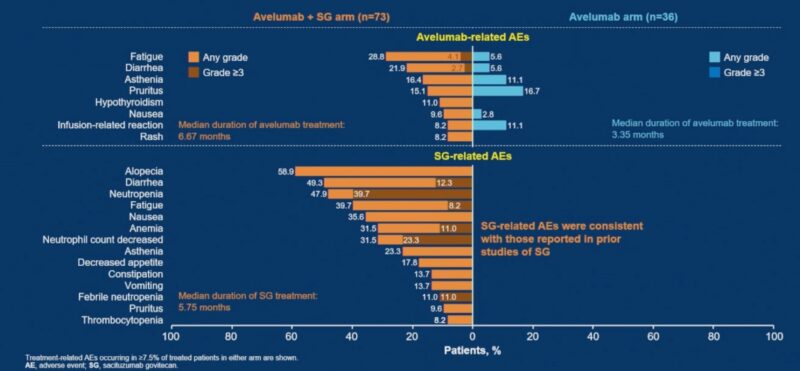
- Safety: Treatment-related adverse events (TRAEs) of any grade occurred in 97.3% of patients receiving avelumab plus SG versus 63.9% with avelumab monotherapy. Grade ≥3 TRAEs were reported in 69.9% of combination patients versus none in the monotherapy arm. TRAEs led to treatment discontinuation in 4.1% of patients in the combination arm (12.3% due to SG alone) and 2.8% in the monotherapy arm. One patient in the combination arm experienced a SG-related adverse event leading to death (acute subarachnoid hemorrhage in the context of sepsis and pancytopenia).
What Are Specialists Saying About the Javelin Trial Results?
#ASCO25 JAVELIN bladder medley phase 2 trial using avelumab and sacituzumab vs avelumab shows a very interesting positive PFS results in mUC, OS is not yet accomplished and suggest a rol of combo antitrop2.
From Dr. Martin Zapata Laguado’s X
Interim analysis of JAVELIN Bladder Medley rand PhII trial of maint avelumab +/- #SG if no PD w/ #1L platinum-chemo for #mUC 7.4 mo PFS benefit & 24% ORR after rand w/#SG vs maint ave alone (n=37 pts from JAVELIN 100 used as extended control), OS immature.
From Dr Jun Gong’s X
Conclusions
In patients with advanced urothelial carcinoma without progression after first-line platinum-based chemotherapy, maintenance treatment with avelumab plus sacituzumab govitecan significantly improved progression-free survival compared to avelumab monotherapy. While treatment-related adverse events were more frequent in the combination arm, they were consistent with the known safety profiles of SG and avelumab. These results support further investigation of avelumab in combination with anti–Trop-2 antibody-drug conjugates in advanced urothelial carcinoma.
You Can Also Read SOHO-01 Trial Results: BAY 2927088 Shows Promising Activity in Advanced HER2-Mutant NSCLC at ASCO 2025 by Oncodaily
Authors
Jeannie Hoffman-Censits, Marinos Tsiatas, Peter Chang, Miso Kim, Lorenzo Antonuzzo, Sang Joon J. Shin, Georgios Gakis, Normand Blais, Se Hyun Kim, Annabel Smith, Jose A. Arranz Arija, Harvey Yu-Li Su, Flora Zagouri, Marco Maruzzo, Christophe Tournigand, Frederic Forget, Astrid Schneider, Karin Tyroller, Natalia Jacob, Begoña P. Valderrama
Affiliations
The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD; Athens Medical Center, Marousi, Greece; Taipei Veterans General Hospital and National Yang Ming Chiao Tung University, Hsinchu, Taiwan; Seoul National University Hospital and College of Medicine, Seoul, South Korea; Careggi University Hospital and University of Florence, Florence, Italy; Yonsei Cancer Center, Seoul, South Korea; Martin-Luther-University of Halle-Wittenberg, Halle, Germany; University of Montreal Health Centre (CHUM), Montréal, Canada; Seoul National University Bundang Hospital, Seongnam, South Korea; Icon Cancer Centre, Adelaide, Australia; Hospital Universitario Gregorio Marañón, Madrid, Spain;
Kaohsiung Chang Gung Memorial Hospital, Taiwan; Alexandra Hospital National and Kapodistrian University of Athens, Greece; Istituto Oncologico Veneto IRCCS, Padova, Italy; Hôpital Henri Mondor, AP-HP, Créteil, France; Centre Hospitalier de l’Ardenne, Libramont-Chevigny, Belgium; Merck KGaA, Darmstadt, Germany; EMD Serono, Billerica, MA; Hospital Universitario Virgen del Rocío, Seville, Spain.
Written by Aharon Tsaturyan , MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
