
DESTINY-Breast09 Trial: T-DXd Plus Pertuzumab Sets a New Benchmark in First-Line HER2-Positive Metastatic Breast Cancer
HER2-positive advanced or metastatic breast cancer (HER2+ a/mBC) remains a clinical challenge, despite major progress over the past decade. Since the landmark CLEOPATRA study, the combination of trastuzumab, pertuzumab, and taxane (THP) has been the established first-line standard of care. The phase 3 DESTINY-Breast09 trial (NCT04784715) was designed to determine whether trastuzumab deruxtecan (T-DXd), an antibody-drug conjugate with proven efficacy in pretreated HER2+ a/mBC, with or without pertuzumab (P), could improve clinical outcomes compared to THP in the first-line setting.

Study Design and Methods
DESTINY-Breast09 is a large, global, randomized phase 3 study enrolling 1,157 patients with centrally confirmed HER2+ (IHC 3+ or ISH+) advanced or metastatic breast cancer. Eligible patients had not received prior chemotherapy or HER2-targeted therapy for metastatic disease; those with prior [neo]adjuvant HER2 therapy or chemotherapy were allowed if their disease-free interval was >6 months. Endocrine therapy for metastatic disease was permitted if limited to one line. Patients were randomized 1:1:1 to receive T-DXd (5.4 mg/kg) plus placebo, T-DXd plus pertuzumab (T-DXd + P), or the standard THP regimen. The primary endpoint was progression-free survival (PFS) by blinded independent central review (BICR); secondary endpoints included overall survival (OS), investigator-assessed PFS, objective response rate (ORR), duration of response (DOR), and safety.
This interim analysis compares T-DXd + P (n=383) versus THP (n=387) after a median follow-up of 29 months, with 38% maturity for PFS events. The T-DXd monotherapy arm remains blinded until final analysis.
Patient Population
The study population was well-balanced between arms, with 52% presenting with de-novo disease and 54% hormone receptor-positive (HR+). Stratification factors included de-novo versus recurrent disease and PIK3CA mutation status.
Efficacy Outcomes
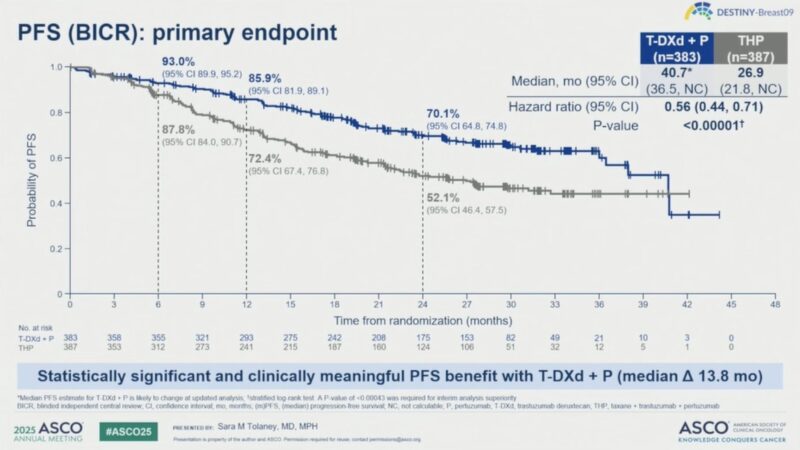
Progression-Free Survival (PFS): T-DXd + P demonstrated a statistically significant and clinically meaningful improvement in PFS compared to THP.
- Median PFS by BICR: 40.7 months (95% CI: 36.5, NC) with T-DXd + P vs 26.9 months (21.8, NC) with THP (HR 0.56; 95% CI 0.44–0.71; P<0.00001).
- 24-month PFS rate: 70.1% (95% CI 64.8–74.8) for T-DXd + P vs 52.1% (46.4–57.5) for THP.
This benefit was consistent across all pre-specified subgroups, including HR status and PIK3CA mutation.
Investigator-Assessed PFS: By investigator assessment, the benefit was even more pronounced:
- Median PFS: 40.7 months (T-DXd + P) vs 20.7 months (THP) (HR 0.49; 95% CI 0.39–0.61).
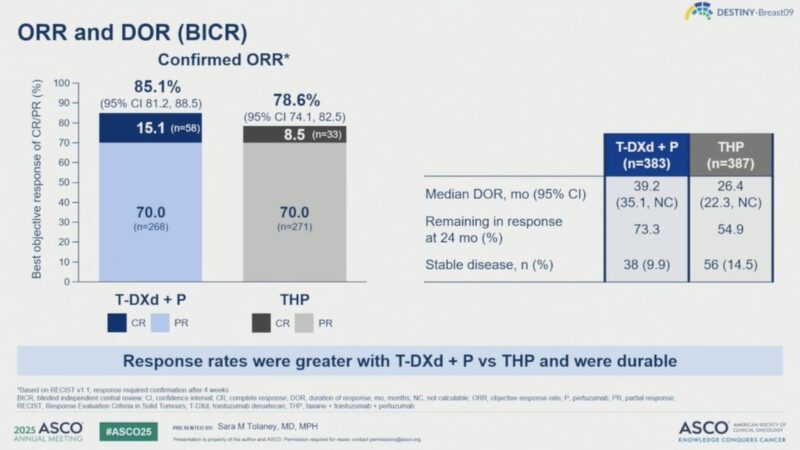
Response Rates and Duration
- Objective Response Rate (ORR) by BICR: 85.1% (95% CI 81.2–88.5) with T-DXd + P vs 78.6% (74.1–82.5) with THP.
- Complete Response Rate: 15.1% with T-DXd + P vs 8.5% with THP.
- Median Duration of Response (DOR): 39.2 months (T-DXd + P) vs 26.4 months (THP).
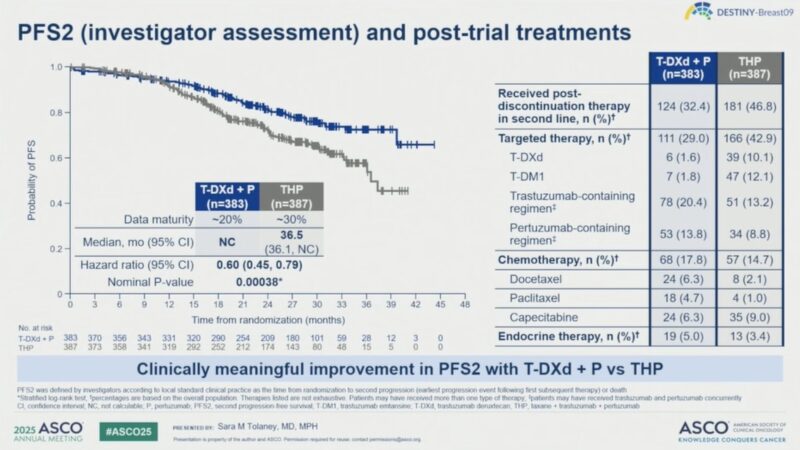
Overall Survival: Overall survival data were immature at the time of this interim analysis, but early trends favored the T-DXd + P arm.
Safety and Tolerability
The overall safety profile was consistent with previous reports for both regimens, with most adverse events manageable and expected based on the drugs’ known toxicity profiles.
- Grade ≥3 treatment-emergent adverse events (TEAEs): 63.5% in T-DXd + P vs 62.3% in THP.
- Serious TEAEs: 27.0% (T-DXd + P) vs 25.1% (THP).
- Interstitial lung disease/pneumonitis: 12.1% (T-DXd + P; mainly grade 1/2, with 0.5% grade 5 events) vs 1.0% (THP; all grade 1/2).
No new safety signals emerged for T-DXd + P.
Conclusions
DESTINY-Breast09 establishes T-DXd plus pertuzumab as a new first-line treatment option for HER2+ advanced or metastatic breast cancer, showing unprecedented median PFS exceeding three years and high objective response rates. The magnitude and consistency of PFS improvement across all subgroups, coupled with a manageable safety profile, strongly support T-DXd + P as a new standard of care. Final overall survival results and additional follow-up will further clarify the long-term benefit.
Clinical Implications
With these findings, the landscape of first-line HER2+ a/mBC management is poised for a significant shift, potentially displacing the decade-long standard of THP. Oncologists and patients now have a new regimen that delivers both extended disease control and a robust safety record.
Key Takeaways
The DESTINY-Breast09 trial demonstrated that T-DXd plus pertuzumab provides a statistically significant and clinically meaningful improvement in progression-free survival compared to the decade-long standard THP regimen for first-line HER2-positive metastatic breast cancer. The regimen achieved an unprecedented median PFS exceeding three years and higher objective response and complete response rates. Safety was manageable and consistent with known profiles, with no new safety signals identified. These results support T-DXd plus pertuzumab as a new standard of care for this patient population.
What They’re Saying: Reactions to DESTINY-Breast09 Trial at ASCO 2025
Luca Arecco, MD, Medical Oncologist from UniGenova, Research Fellow at JulesBordet Institute shared on X
Presented by stolaney1, the awaited results of DESTINY-Breast09 trial for first-line, HER2+ mBC
PFS: T-DXd+P: 40.7 mo vs 26.9 mo THP (HR 0.56), Delta of: 13.8 mo!, ILD any G: 12.1% T-DXd vs 1% THP, New SOC in 1L HER2+ BC setting after >10y!
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
