
Amol Akhade: EMBER-3 Trial Results in ER+/HER2- Metastatic Breast Cancer
Amol Akhade, Senior Consultant Medical Oncologist and Hemato-Oncologist at Suyog Cancer Clinics and Reliance Hospitals, shared a post on LinkedIn:
“Big news from ESMOBreast25:
The EMBER-3 trial has delivered the first Phase 3 success for an oral SERD + CDK4/6i combo in the post-CDK4/6 setting for ER+/HER2- metastatic breast cancer (MBC). This could reshape the therapeutic landscape for patients progressing on first-line therapy.
Trial Summary: EMBER-3
Patients: ER+/HER2- MBC, previously treated with CDK4/6 inhibitors.
Treatment arms:
- Imlunestrant and Abemaciclib vs
- Imlunestrant alone.
Progression-Free Survival (PFS) Gains.
ESR1-mutant tumors:
– 11.1 vs 5.5 months | HR 0.44
PI3K-mutant tumors:
– 7.5 vs 3.7 months | HR 0.52
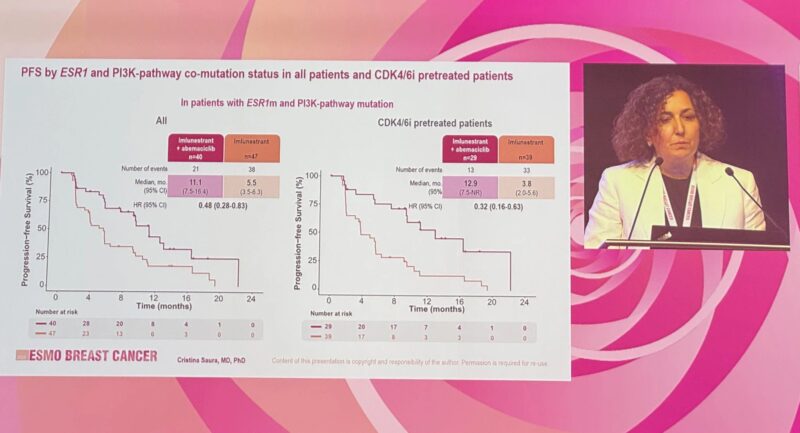
ESR1 + PI3K co-mutant:
– 12.9 vs 3.8 months | HR 0.32
All comers:
– 9.4 vs 5.5 months | HR 0.57
CDK4/6i pretreated subset:
– 9.1 vs 3.7 months | HR 0.51
Why This Matters:
- All-oral regimen = patient convenience.
- Consistent benefit in biomarker-positive and negative subgroups.
- Addresses unmet need post-CDK4/6 failure.
- Potential to become a new standard for second-line ER+ MBC.
But Let’s Be Critical:
- Comparator was Imlunestrant monotherapy, not standard options like fulvestrant or chemotherapy.
- Overall survival (OS) data is still immature.
- Sample sizes in mutation-defined subgroups (e.g., ESR1+, PI3K+) were relatively small.
- We still need clarity on long-term endocrine sensitivity and resistance mechanisms.
Final Thought:
The EMBER-3 trial is a pivotal step forward in post-CDK4/6 endocrine therapy.
While more data is needed to confirm overall survival benefit, the early PFS signals are promising, especially in ESR1-mutant and PI3K-mutant disease—often the toughest to treat.
Keep an eye on oral SERDs as they carve a new niche in ER+ MBC treatment algorithms.”
The EMBER-3 trial marks the first Phase 3 success of an oral SERD and CDK4/6 inhibitor combination in ER+/HER2- metastatic breast cancer post-CDK4/6 therapy. Results suggest a significant progression-free survival benefit across key biomarker subgroups. Limitations include monotherapy comparators and immature OS data.
More posts featuring Amol Akhade.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
