
Immunohistochemistry (IHC): How Antibodies Help Diagnose and Classify Disease
Immunohistochemistry, often abbreviated as IHC, is a powerful laboratory technique that plays a central role in modern medicine. This technique is especially valuable when diagnosing tumors, as it helps identify what kind of cells are involved, how aggressive they may be, and whether they might respond to certain treatments. IHC also plays an important role in detecting infections, immune-related diseases, and even neurodegenerative conditions.
In this article, we’ll take a closer look at how IHC works, its key applications in cancer care, some of the most commonly used markers, the benefits and challenges of the method, and how it is increasingly being used in personalized and targeted medicine. Through this lens, you’ll see why immunohistochemistry has become one of the most indispensable tools in pathology today.
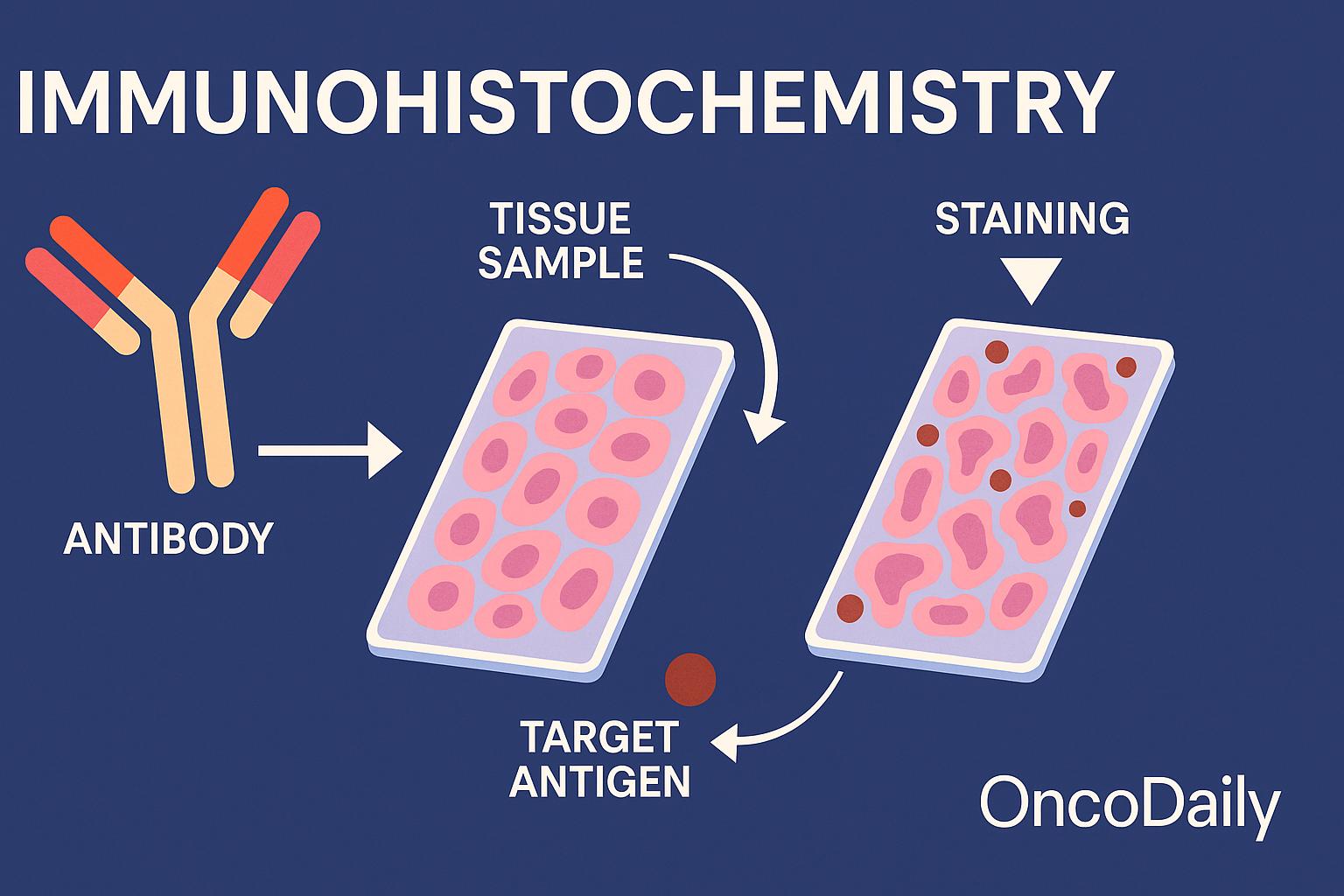
How Immunohistochemistry Works
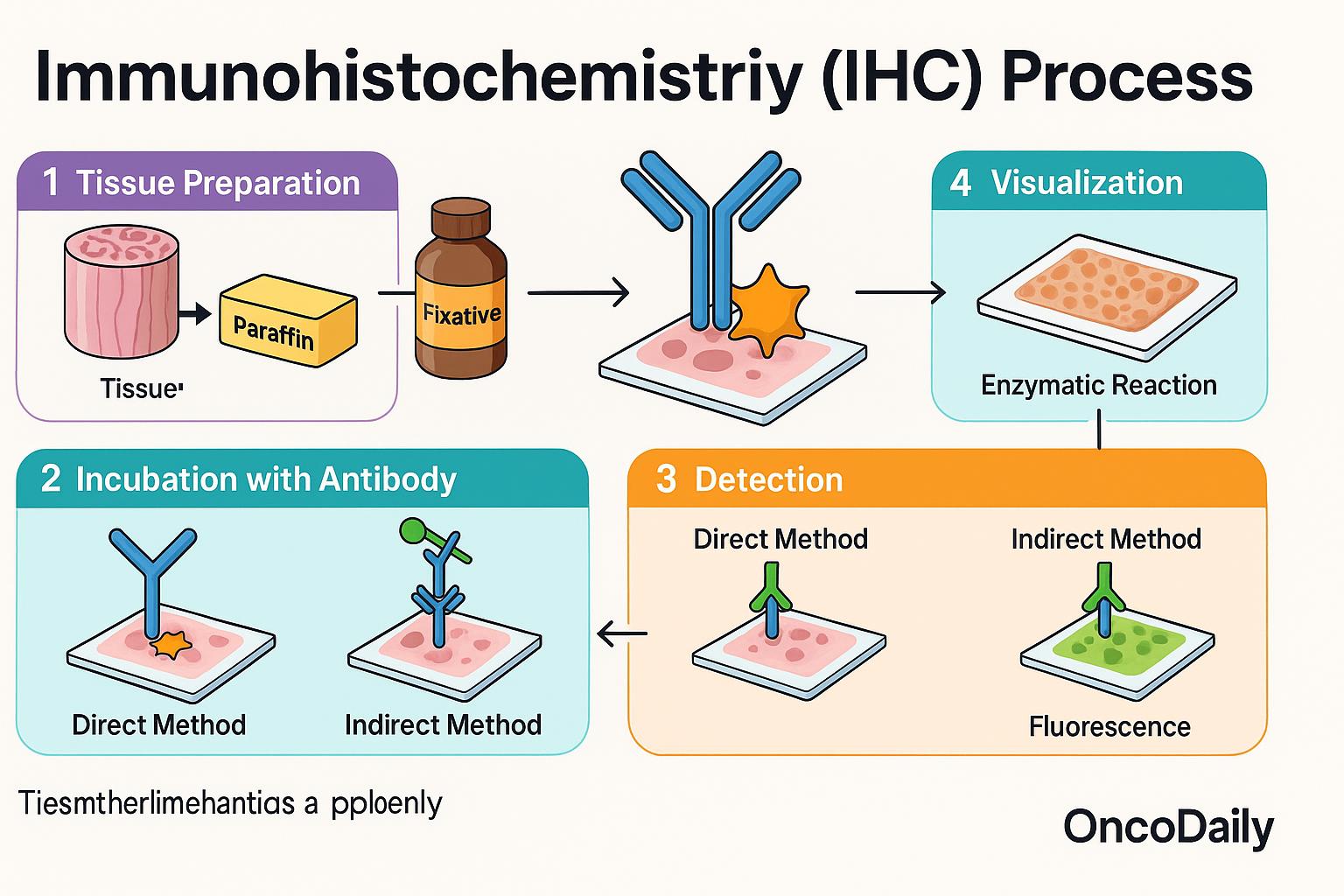
The immunohistochemistry (IHC) process begins with a tissue sample—usually obtained through a biopsy or surgical removal—which is preserved using a chemical fixative like formalin and then embedded in paraffin wax to maintain its structure. This preserved tissue is sliced into very thin sections and placed onto microscope slides for analysis.
Next, specially prepared antibodies are applied to the tissue. These antibodies are designed to bind only to a specific protein—known as an antigen—that the pathologist wants to detect. If the protein is present in the tissue, the antibody will bind to it. To make this interaction visible, a detection system is added that produces a color change, often appearing as a brown (commonly using a DAB chromogen) or red stain, allowing the protein’s location and abundance to be seen under the microscope. There are two main methods used to visualize this antibody-antigen binding:
- In the direct method, the primary antibody is directly linked to the detection molecule (such as an enzyme or fluorescent tag).
- In the indirect method, which is more commonly used, a second antibody is used to detect the primary antibody. This amplifies the signal and increases sensitivity, making it easier to detect even small amounts of protein.
The final step involves visualization, which can be achieved using enzymatic reactions (e.g., DAB producing a brown stain) or fluorescent labeling, where the tissue is viewed under a fluorescence microscope. This entire process allows pathologists to evaluate protein expression patterns, which are critical for diagnosing diseases and guiding treatment decisions.
Applications of IHC in Medicine
Immunohistochemistry (IHC) is widely used in medicine for its ability to provide detailed insight into tissue biology, and it plays a particularly vital role in the diagnosis and classification of cancer. One of its most essential uses is to help pathologists distinguish between benign and malignant tumors, and to identify the tissue of origin, especially in cases where cancer has metastasized and the primary source is unknown. By examining the patterns of protein expression, IHC also contributes to the grading (how aggressive the tumor appears) and staging (how far it has spread) of many cancers.
Beyond diagnosis, IHC is used to identify prognostic markers—proteins that provide information about how a tumor is likely to behave. For example, Ki-67 is a marker of cell proliferation; high levels may indicate a fast-growing tumor. Proteins like p53, a tumor suppressor, or BCL-2, which helps cells avoid programmed death, can also provide clues about tumor biology and patient prognosis.
IHC is equally important in identifying predictive markers, which indicate whether a tumor is likely to respond to specific targeted therapies. For instance, HER2 testing in breast cancer helps determine whether a patient will benefit from trastuzumab (Herceptin). PD-L1 testing is used in lung and other cancers to assess eligibility for immunotherapy, and hormone receptor status (ER/PR) guides the use of endocrine therapy in breast cancer. Outside of oncology, IHC is used to detect proteins produced by infectious organisms in tissue samples. For example, it can identify viruses like cytomegalovirus (CMV) or human papillomavirus (HPV), and bacteria like Mycobacterium tuberculosis in infected tissue.
In neurodegenerative diseases, IHC is used to detect abnormal protein accumulations, such as tau and amyloid-beta, which are hallmarks of Alzheimer’s disease and other forms of dementia. This application has become increasingly important in both clinical diagnosis and research into brain disorders. Altogether, IHC is a versatile and indispensable tool across medical specialties, offering insights that shape diagnosis, prognosis, and treatment strategies.
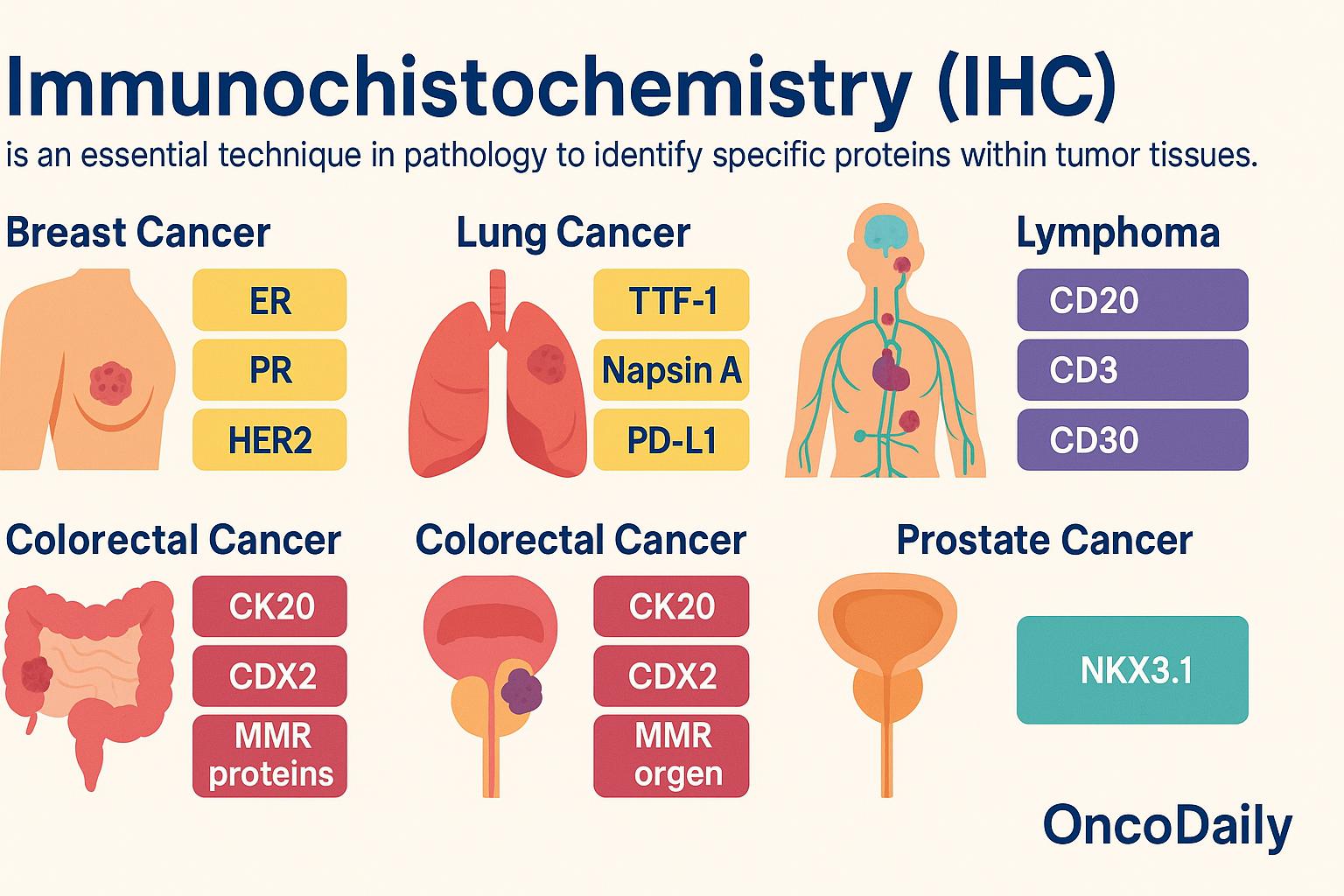
Common IHC Markers in Oncology
Immunohistochemistry (IHC) is an essential technique in pathology that allows clinicians to identify the presence and distribution of specific proteins within tumor tissues. These protein markers help determine the type of cancer, confirm its site of origin, and guide treatment decisions.
In breast cancer, the most commonly evaluated markers include estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67. ER and PR help determine whether the tumor is hormone-sensitive and likely to respond to endocrine therapy. HER2 is crucial for identifying patients who may benefit from HER2-targeted therapies like trastuzumab. Ki-67 provides a measure of how rapidly the tumor cells are dividing, which helps assess aggressiveness. For lung cancer, IHC is used to detect markers such as TTF-1 and Napsin A, which support the diagnosis of primary lung adenocarcinoma. PD-L1 expression, also assessed by IHC, is important in evaluating whether a patient may benefit from immune checkpoint inhibitors like pembrolizumab.
In the diagnosis of lymphoma, IHC is indispensable for subclassification. CD20 identifies B-cell lineage and guides the use of anti-CD20 therapies like rituximab. CD3 marks T-cell lineage, while CD30 is frequently expressed in Hodgkin lymphoma and certain types of T-cell lymphomas, offering a target for brentuximab vedotin. Ki-67 is again used here to assess proliferation index and tumor aggressiveness. For colorectal cancer, IHC markers such as CK20 and CDX2 help confirm gastrointestinal origin. Mismatch repair (MMR) proteins—including MLH1, MSH2, MSH6, and PMS2—are evaluated to determine microsatellite stability. Loss of one or more of these proteins suggests microsatellite instability (MSI), which has implications for prognosis, immunotherapy response, and screening for Lynch syndrome.
In prostate cancer, NKX3.1 is a highly specific IHC marker that helps confirm prostate origin, particularly in metastatic tumors where other markers may be less reliable. By using panels of these IHC markers, pathologists can construct a precise tumor profile that not only confirms the diagnosis but also provides critical information to tailor treatment—making immunohistochemistry a cornerstone of modern oncology.
Advantages of IHC
One of the key strengths of immunohistochemistry (IHC) is its ability to deliver highly specific and sensitive results. Antibodies used in IHC are designed to bind to unique protein targets, which allows for the accurate identification of specific cell types, tumor markers, or disease-related proteins—even in complex tissue environments.
Another major advantage is that IHC preserves the tissue architecture. Unlike molecular tests that analyze extracted DNA or RNA, IHC allows the pathologist to observe the exact location of protein expression within the intact tissue. This means the pathologist can not only determine whether a marker is present, but also see where in the cell it is located—such as in the nucleus, cytoplasm, or cell membrane. This spatial context can provide critical diagnostic and prognostic information.
IHC is also well-suited for use on formalin-fixed, paraffin-embedded (FFPE) tissue samples, which are the standard method for preserving biopsy and surgical specimens. This makes IHC widely accessible and routinely applicable in clinical pathology labs around the world. With its combination of precision, versatility, and compatibility with standard tissue processing methods, IHC remains one of the most valuable tools in diagnostic medicine.
Limitations and Challenges
While immunohistochemistry (IHC) is a powerful diagnostic tool, it is not without its limitations. One of the primary challenges is that interpreting IHC results requires significant expertise. The process involves visually assessing the intensity and pattern of staining under a microscope, which can sometimes be subjective. Two different pathologists may occasionally draw different conclusions, especially in borderline or weakly stained cases.
There’s also the risk of false positives or false negatives. A false positive can occur if an antibody binds non-specifically to a different protein, while a false negative might result from poor tissue preservation or low antigen expression. These issues can lead to diagnostic errors if not carefully controlled. Another critical factor is the quality of antibodies and variability in laboratory protocols. Differences in antibody clones, concentrations, incubation times, and detection systems can all impact the accuracy and reproducibility of IHC results across laboratories. Standardization is essential but not always achieved uniformly.
Finally, not all markers are entirely specific to one tissue type or disease. For instance, CD10 can be expressed in both renal cell carcinoma and certain lymphomas, which can complicate interpretation if used in isolation. That’s why IHC is often performed using panels of markers, rather than relying on a single antibody, to improve diagnostic accuracy and avoid misclassification. Despite these challenges, when used carefully and interpreted by skilled pathologists, IHC remains a highly reliable and informative method in diagnostic pathology.
IHC and Personalized Medicine
Immunohistochemistry (IHC) plays a crucial role in the era of personalized medicine, helping guide treatment decisions by revealing specific molecular characteristics of tumors. One of its most impactful uses is in selecting targeted therapies. For example, in HER2-positive breast cancer, IHC is used to measure HER2 protein overexpression. If the tumor tests positive, patients may benefit from trastuzumab (Herceptin) or other HER2-targeted drugs, which can significantly improve outcomes. IHC is also instrumental in predicting response to immunotherapy. A key example is the assessment of PD-L1 expression in cancers such as non-small cell lung cancer, melanoma, and bladder cancer. Patients with high PD-L1 expression may be eligible for immune checkpoint inhibitors like pembrolizumab, which harness the immune system to fight cancer.
Moreover, IHC is increasingly being integrated with molecular testing, such as next-generation sequencing (NGS), to provide a more comprehensive view of a tumor’s biology. While molecular testing detects genetic mutations and alterations at the DNA level, IHC complements this by showing how these changes translate into actual protein expression within the tumor tissue. Together, these tools enable more precise classification, better risk stratification, and tailored treatment strategies based on the individual characteristics of each patient’s cancer.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What is immunohistochemistry (IHC)?
Immunohistochemistry is a laboratory technique that uses antibodies to detect specific proteins in tissue samples, aiding in disease diagnosis and classification.
How is IHC used in cancer diagnosis?
IHC helps identify the type of cancer, its origin, aggressiveness, and potential response to targeted therapies.
What are the most common IHC markers in cancer?
Common markers include ER, PR, HER2, Ki-67 (breast cancer), PD-L1 (lung and others), CD20, CD3 (lymphoma), and mismatch repair proteins (colorectal cancer).
What is the difference between direct and indirect IHC methods?
Direct IHC uses a labeled primary antibody, while indirect IHC uses a secondary antibody to amplify the signal from the primary antibody.
How reliable is IHC?
IHC is generally accurate but can be affected by antibody quality, sample preparation, and interpretation variability.
Can IHC detect infections?
Yes, IHC can detect viral and bacterial proteins, such as HPV or Mycobacterium tuberculosis, in tissue.
What are the limitations of IHC?
Limitations include potential false positives/negatives, non-specific staining, and variability in interpretation among pathologists.
What role does IHC play in personalized medicine?
IHC identifies biomarkers like HER2 or PD-L1 to guide targeted therapies, making it vital for personalized cancer treatment.
Is IHC used only for cancer diagnosis?
No. IHC is also used to diagnose infectious, autoimmune, and neurodegenerative diseases.
How are IHC results interpreted?
A pathologist examines stained tissue under a microscope to assess protein expression levels and patterns, aiding diagnosis and treatment planning.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
