
Vimseltinib (Romvimza): Uses in Cancer, Side effects, Dosage, Expectations, and more
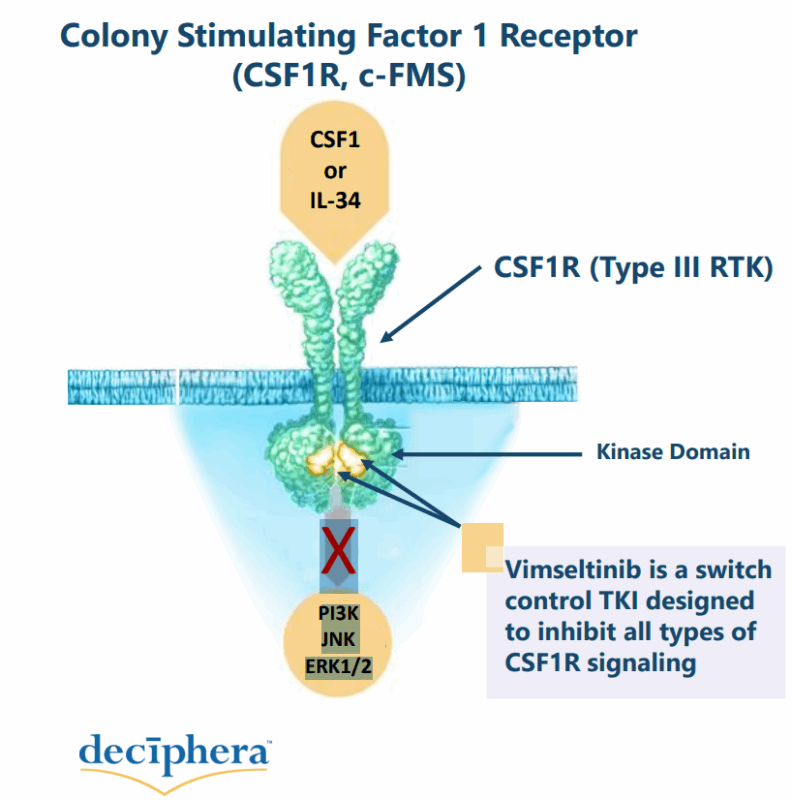
Vimseltinib is an oral kinase inhibitor that selectively targets the colony-stimulating factor 1 receptor (CSF1R).
Unlike other CSF1R inhibitors, vimseltinib is designed as a switch-control inhibitor, meaning it binds to a unique regulatory region of CSF1R to provide prolonged target inhibition with reduced off-target effects.
Vimseltinib was approved by the U.S. Food and Drug Administration (FDA) on February 14, 2025. It is indicated for the treatment of adult patients with symptomatic tenosynovial giant cell tumor (TGCT) when surgical resection is likely to worsen functional limitation or severe morbidity.
Which company produced Vimseltinib?
Vimseltinib, marketed under the brand name Romvimza, was developed by Deciphera Pharmaceuticals, a biotechnology company headquartered in Waltham, Massachusetts. In April 2024, Deciphera became a wholly owned subsidiary of Ono Pharmaceutical Co., Ltd., following a $2.4 billion acquisition. Deciphera specializes in precision oncology, focusing on the development of small-molecule inhibitors that target key cancer-driving proteins. Its research pipeline includes vimseltinib for the treatment of tenosynovial giant cell tumor, along with other investigational therapies. Romvimza continues to be developed and commercialized by Deciphera Pharmaceuticals.
How does Vimseltinib work?
Vimseltinib is a selective CSF1R inhibitor that blocks the overactive CSF1R signaling in TGCT. In healthy tissue, the colony-stimulating factor 1 receptor (CSF1R) regulates the growth and function of certain immune cells called macrophages, which are important for the immune response and tissue repair. CSF1R is activated by binding to its ligands, colony-stimulating factor 1 (CSF1) and interleukin-34 (IL-34), triggering signaling pathways that promote the survival and activation of these immune cells.
It works by binding to the CSF1R receptor, preventing the activation of downstream signaling pathways that promote tumor growth and macrophage proliferation. By inhibiting CSF1R, vimseltinib reduces tumor size and symptoms in patients with TGCT, offering a targeted treatment for a disease that is difficult to manage with surgery alone.
What Cancers Is Vimseltinib Approved to Treat?
Romvimza was FDA approved on February 14, 2025, for the treatment of symptomatic tenosynovial giant cell tumor (TGCT) in adult patients when surgery is not a suitable option due to the risk of severe morbidity or functional limitations.
Currently, TGCT is the only cancer for which vimseltinib has received regulatory approval. However, it is being investigated for potential use in other cancers involving CSF1R-driven tumor growth.
What research is behind the approval?
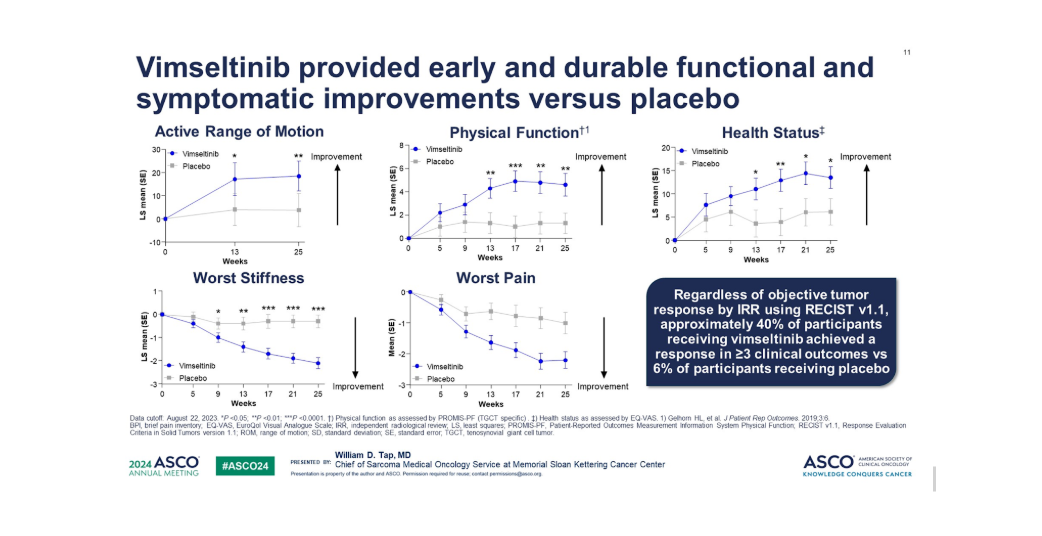
A study published in JCO on May 29, 2024, reported findings from the MOTION trial, a global phase 3 study evaluating vimseltinib, a selective CSF1R inhibitor, in symptomatic tenosynovial giant cell tumor (TGCT) not suitable for surgery. In this randomized (2:1), double-blind trial, 123 patients received either vimseltinib (30 mg twice weekly) or a placebo for 24 weeks. At week 25, vimseltinib demonstrated significantly higher objective response rates (ORR) compared to placebo (40% vs 0% per RECIST v1.1, 67% vs 0% per tumor volume score; P < 0.0001).
Patients treated with vimseltinib also experienced greater improvements in range of motion, physical function, stiffness, pain, and overall health status (P < 0.05). The drug was well tolerated, with mostly grade 1/2 adverse events and no liver toxicity concerns. These findings support vimseltinib as an effective and well-tolerated therapy for TGCT, offering significant clinical benefits in tumor reduction, function, and quality of life.
Read about the FDA approval of Vimseltinib (Romvimza) for symptomatic Tenosynovial Giant Cell Tumor (TGCT) on OncoDaily.
What is Tenosynovial Giant Cell Tumor?
Tenosynovial Giant Cell Tumor (TGCT) is a rare, typically benign tumor that affects the synovium—the thin layer of tissue lining joints and tendons. It is characterized by the proliferation of synovial cells, leading to tumor formation. TGCT is classified into two main types:
- Localized TGCT (Giant Cell Tumor of Tendon Sheath): This form usually presents as a slow-growing, painless mass, most commonly in the fingers or hands. It’s the second most common tumor of the hand, following ganglion cysts.
- Diffuse TGCT (Pigmented Villonodular Synovitis – PVNS): This type is less common and involves more extensive joint involvement, often affecting the knee. It can cause joint swelling, pain, and stiffness, potentially leading to joint damage if untreated.
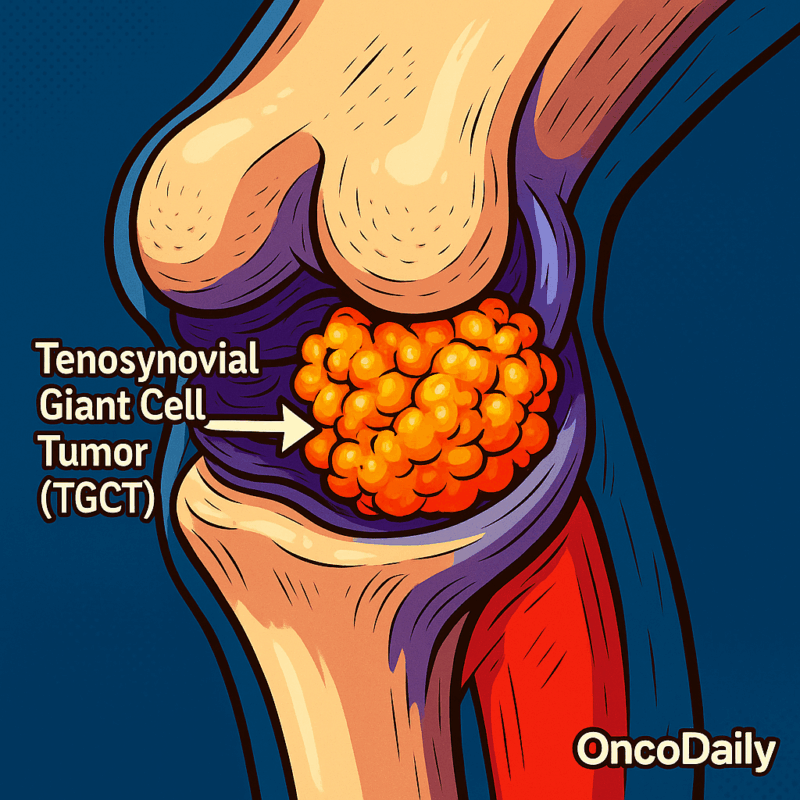
While benign and not cancerous, they can cause significant damage to surrounding tissue and limit joint movement.
TGCT is characterized by a chromosomal translocation involving chromosomes 1 and 2, leading to an overproduction of colony-stimulating factor-1 (CSF-1), which recruits macrophages to form the tumor. The exact cause of this translocation is unclear, and there are no known risk factors for developing TGCT.
Most affected individuals are between 25-50 years old, with slightly more females affected in localized TGCT. The global incidence is estimated at 43 cases per 1 million people, with localized TGCT being more common.
Symptoms of Tenosynovial Giant Cell Tumor
Vary but often include pain, swelling, tenderness, and joint instability. TGCT can present in two forms: localized, which affects smaller joints like the fingers or feet, and diffuse, which usually involves larger joints like the knee. Surgery is often the first treatment for TGCT, but tumors can recur, especially in the diffuse form. Symptoms can resemble other conditions, such as synovial hemangiomas or sports injuries, requiring careful diagnosis. Diagnostic tools include X-rays, MRIs, and sometimes biopsies.
Treatment options for Tenosynovial Giant Cell Tumor
Treatment for TGCT typically involves surgery, but recurrence is common, especially in diffuse TGCT. In some cases, radiation therapy or drugs like pexidartinib (Turalio) and imatinib (Gleevec) may be used, particularly when surgery isn’t feasible or the tumor recurs.
Vimseltinib combinations and treatment outcomes
As of April 2025, vimseltinib has been primarily studied as a monotherapy for tenosynovial giant cell tumor (TGCT) and some advanced solid tumors. Current trials, including the MOTION study, focus on its efficacy and safety when used alone. There is no available data on vimseltinib being tested in combination with other therapies, though future research may explore this approach.
Vimseltinib side effects and their management
Vimseltinib is an effective treatment for tenosynovial giant cell tumor (TGCT), a rare and aggressive tumor. While it offers significant clinical benefits, like all medications, it can lead to side effects. Most patients tolerate the drug well, but some may experience mild to moderate adverse reactions. Regular monitoring and prompt management of these side effects are essential to ensure the best outcomes.
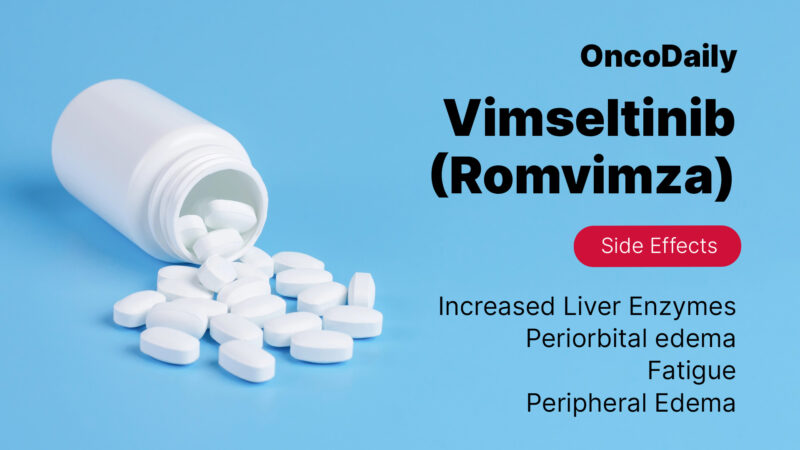
Common side effects
Some of the most common adverse effects include increased liver enzymes, periorbital edema (swelling around the eyes), fatigue, rash, elevated cholesterol levels, peripheral edema, decreased white blood cell counts, and pruritus (itching). Patients receiving Romvimza may require regular liver function monitoring, as elevated AST and ALT levels can occur, sometimes necessitating dose adjustments or treatment discontinuation. Periorbital and peripheral edema can be managed by temporary dose reductions, leg elevation, or salt intake reduction, while fatigue may improve with balanced activity and rest.
Skin-related reactions, including rash and pruritus, are typically mild and can be controlled with antihistamines, topical corticosteroids, or moisturizers. Elevated cholesterol levels may require dietary modifications or lipid-lowering medications, and reduced white blood cell counts should be monitored through routine blood tests to assess the need for dose modifications.
Less common side effects
Less commonly, vimseltinib may cause gastrointestinal disturbances such as diarrhea, nausea, or vomiting, which can often be managed with hydration and symptomatic medications. Some patients report headaches or muscle and joint pain, which may respond to over-the-counter pain relievers or physical therapy. Hypertension has also been noted in some cases, requiring regular blood pressure monitoring and possible antihypertensive treatment.
Overall, Romvimza has shown a favorable safety profile, with most side effects being mild to moderate in severity. Regular medical monitoring and early symptom management can help minimize complications. Patients should be encouraged to communicate any concerning symptoms to their healthcare providers for appropriate intervention.
What is the Recommended Dosage of Vimseltinib?
Vimseltinib is available in capsule form in strengths of 14 mg, 20 mg, and 30 mg. It is indicated for the treatment of symptomatic tenosynovial giant cell tumor (TGCT) in cases where surgery may lead to significant functional limitations or severe morbidity. The recommended dose is 30 mg orally, taken twice a week.
How is Vimseltinib administered?
Vimseltinib should be taken orally and can be administered with or without food. Patients are advised to swallow the capsules whole without opening, breaking, or chewing them. Doses should be spaced by at least 72 hours. Patients need to follow the schedule provided on the blister packaging and take their doses on the same days each week.
If a dose is missed and it’s within 48 hours, patients should take the missed dose as soon as possible and continue with the next dose on the regularly scheduled day. If more than 48 hours have passed, the missed dose should be skipped, and the next dose should be taken on the usual day. In case of vomiting within 30 minutes of taking a dose, patients should repeat the dose, but if it’s been more than 30 minutes, they should take the next dose on the scheduled day.
Romvimza should be stored at room temperature 20-25ºC, with brief excursions allowed between 15-30ºC. The capsules should remain in the original blister packs until they are ready to be taken and should not be stored in another container.
What to Avoid During Vimseltinib Treatment?
During treatment with vimseltinib, there are several precautions that patients should be aware of to ensure both safety and the effectiveness of the drug. First, it is important to avoid consuming grapefruit and grapefruit juice, as they may interfere with the metabolism of vimseltinib, increasing the risk of side effects. Patients should also make sure to swallow the capsules whole, as breaking or chewing them can alter the drug’s intended release and effectiveness.
It is essential to follow the prescribed dosing schedule, as skipping doses or taking them too closely together could reduce the drug’s effectiveness and increase the risk of side effects. Additionally, alcohol consumption should be minimized or avoided, as it can increase the risk of liver toxicity and exacerbate side effects such as dizziness or nausea.
Vimseltinib and pregnancy
Vimseltinib is contraindicated in pregnancy as it may harm the developing fetus. Patients should use effective contraception during treatment and for a period after completing the therapy. Finally, patients should inform their healthcare provider of any other medications they are taking, including over-the-counter drugs and supplements, as some may interact with vimseltinib and either enhance side effects or reduce its effectiveness.
Vimseltinib effectiveness over time
Vimseltinib has shown significant effectiveness in clinical trials, particularly for symptomatic tenosynovial giant cell tumor (TGCT). In the MOTION trial, patients treated with vimseltinib experienced meaningful improvements in tumor response, range of motion, pain reduction, and quality of life within the first 25 weeks. The drug demonstrated a higher objective response rate (ORR) compared to placebo, indicating its potential to reduce tumor size and symptoms over time. While early results are promising, further long-term data is needed to confirm its effectiveness beyond the initial treatment period.
Ongoing trials with Vimseltinib
The study, titled A Phase 2, Open-label, Multicenter Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Vimseltinib in Adults With Active Chronic GVHD After Failure of Prior Systemic Therapy (NCT06619561), aims to evaluate the safety, tolerability, pharmacokinetics, and efficacy of vimseltinib in adults suffering from active, moderate to severe chronic graft-versus-host disease (cGVHD) who have not responded to prior systemic treatments. Participants will be treated with vimseltinib in 28-day cycles for up to two years, and the study’s objective is to determine whether vimseltinib can offer an effective treatment option for these patients.
FAQ
What is Romvimza used for?
Vimseltinib is used to treat symptomatic tenosynovial giant cell tumor (TGCT) in adults when surgery is not suitable due to the risk of severe complications.
How does Vimseltinib work?
Vimseltinib blocks CSF1R, a receptor that contributes to tumor growth in TGCT, reducing tumor size and symptoms.
Who produces Vimseltinib?
Vimseltinib is produced by Deciphera Pharmaceuticals, a biotech company specializing in cancer therapies.
What are the most common side effects of Vimseltinib?
Common side effects include fatigue, rash, swelling around the eyes, elevated liver enzymes, and elevated cholesterol levels.
How is Vimseltinib taken?
Vimseltinib is taken orally as capsules, with a dose of 30 mg twice a week. It should be swallowed whole.
What should patients avoid while taking Vimseltinib?
Patients should avoid grapefruit and alcohol and should not break or chew the capsules
What is TGCT, and how is it treated?
TGCT is a rare tumor affecting the synovium. Treatment usually involves surgery, but Vimseltinib offers an alternative for cases where surgery is not feasible.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
