Essential Read: Hallmarks of Precancer on Cancer Discovery
The Hallmarks of Precancer by Stangis et al. published in Cancer Discovery (14(4): 683-689) establishes key principles governing early lesions, providing crucial insights into cancer initiation and progression. The study emphasizes that early intervention, before complexity develops, is key to effectively combating cancer. Identifying progression risk factors represents a critical aspect of intervention with immediate impact potential.
Title: The Hallmarks of Precancer
Authors: Mary M. Stangis, Zhengyi Chen, Jimin Min, Sarah E. Glass, Jordan O. Jackson, Megan D. Radyk, Xen Ping Hoi, W. Nathaniel Brennen, Ming Yu, Huy Q. Dinh, Robert J. Coffey, Martha J. Shrubsole, Keith S. Chan, William M. Grady, Srinivasan Yegnasubramanian, Costas A. Lyssiotis, Anirban Maitra, Richard B. Halberg, Neelendu Dey, Ken S. Lau
Background and Significance
Cancer remains a leading cause of death globally despite advances in screening and treatment. Understanding precancerous states offers tremendous potential for early intervention, as demonstrated by the substantial decline in cervical cancer following systematic screening and preventative vaccination. Precancers represent a critical spectrum ranging from at-risk normal-appearing tissues to overt lesions from which cancers develop. While appearing normal, precancerous tissues possess distinct molecular properties that differentiate them from both normal and cancerous tissues.
Key Challenges in Precancer Research
The authors highlight several significant challenges in studying precancers:
- Progression identification: Only a small percentage of precancers advance to malignancy
- Trajectory tracking: Following specific precancerous lesions is difficult as standard care mandates complete removal
- Independent events: Asynchronous lesions from the same patient are genetically independent
Six Hallmarks of Precancer
The researchers present a striking framework—six universal hallmarks that define the biology of precancerous states:
Genetic Aging
Telomere shortening and accumulation of somatic mutations, even in histologically normal tissue.
Epigenetic Drift
Specific aberrant methylation patterns accelerating a “biological clock” measurable by tools like the Horvath clock.
Metabolic Reprogramming
Early metabolic shifts favoring glycolysis over oxidative phosphorylation—similar to the Warburg effect seen in cancer.
Regenerative Hijacking
Abnormal activation of stem-like states or metaplasia, turning wound-healing into a pro-tumor process.
Immune Surveillance Collapse
Gradual loss of cytotoxic T cells and natural killer (NK) cell functions, tipping the balance toward immune evasion.
Microenvironmental Remodeling
Senescent fibroblasts and stromal reprogramming create physical barriers and inflammatory environments that nurture precancer.
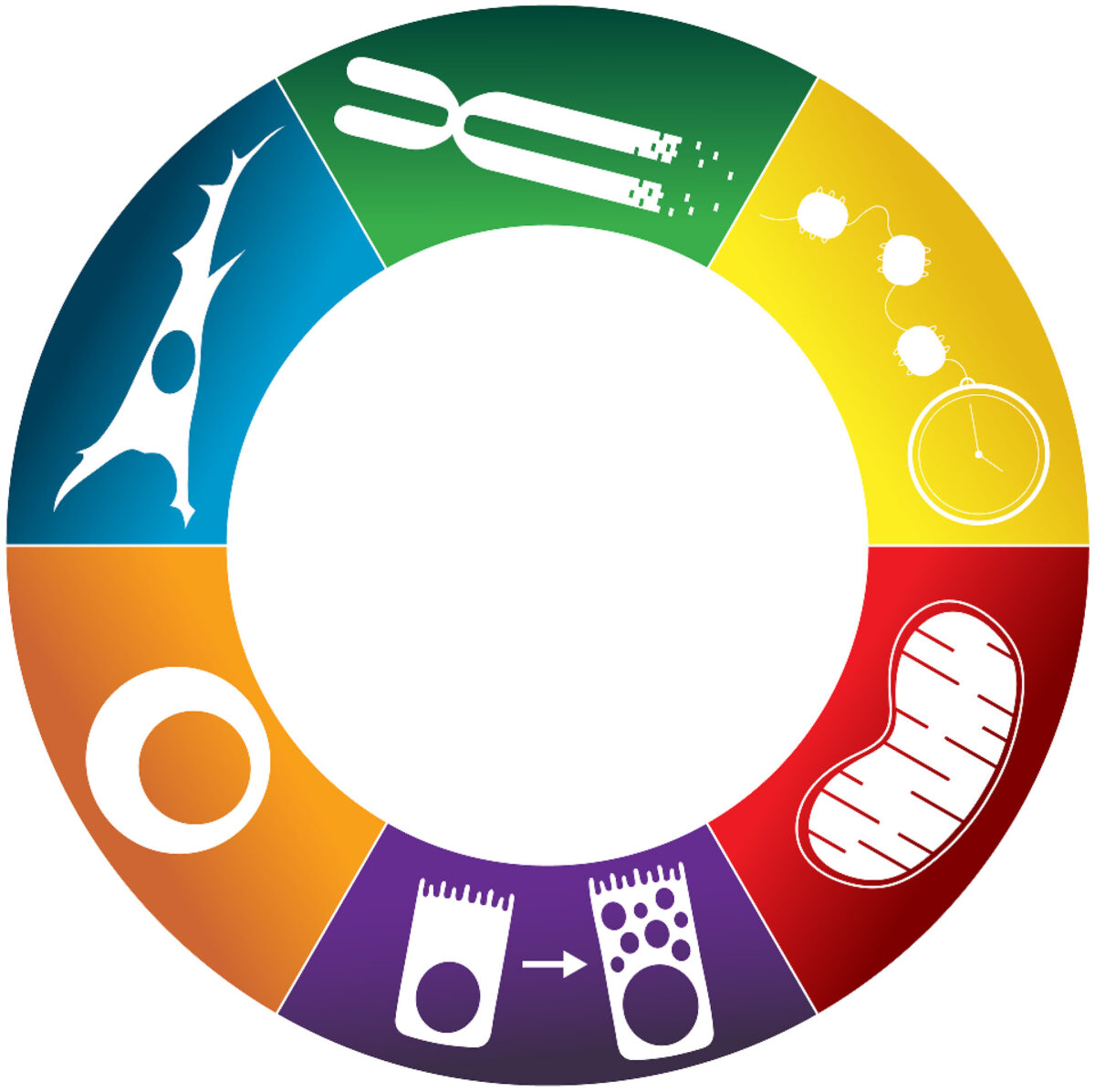
Figure 1. The hallmarks of precancer.Starting at the top center wedge of the ring and proceeding clockwise, these are: (1) age-related genetic alterations including telomere shortening and somatic mutations; (2) epigenetic changes resulting from aberrant methylation at specific sites; (3) metabolic alterations; (4) hijacking of regenerative cell state transitions; (5) disruption of immune surveillance and “inflammaging”; and (6) remodeling of the tissue microenvironment mediated by senescent fibroblasts.
Age Is More Than Just a Number
Chronological age remains a blunt instrument. Biological aging, marked by molecular scars like telomere shortening and DNA mutations, offers much better predictive power.
Shockingly, over 50% of the mutations seen in cancers occur during the precancerous phase, long before clinical detection. These mutations can clonally expand, silently occupying large regions of tissue. Moreover, epigenetic aging clocks show that even “normal” tissues in different racial groups, like African Americans’ right-sided colon tissues, age differently at the molecular level—mirroring real-world disparities in cancer incidence.
It’s not just the number of birthdays you’ve had, but the molecular wear-and-tear on your tissues that matters.
Macroenvironmental Risk Factors
Today’s global rise in early-onset cancers strongly suggests that environmental and lifestyle factors are accelerating biological aging.
-
Western diet (high in sugar, fat, and processed foods)
-
Obesity (hyperinsulinemia fosters tumor growth via the PI3K pathway)
-
Microbiome disruption (loss of protective microbial diversity)
-
Social determinants (food deserts, healthcare inequality, transportation access)
For instance, vitamin C deficiency, linked to food insecurity, speeds up leukemogenesis by impairing TET2—a key tumor suppressor. Clearly, biological aging and environmental exposures compound each other, making some individuals dramatically more susceptible to precancer—and explaining part of the rise in early-onset cancers.
When Healing Goes Wrong or Regenerative Hijacking
Cells exposed to chronic injury, inflammation, or metabolic stress sometimes try to repair themselves by reverting to a stem-like state. This “wound that never heals” model reveals that:
-
Normal regenerative programs become hijacked by oncogenic pathways.
-
Stem cell proliferation increases mutation accumulation.
-
Metaplasia—replacement of one cell type by another—becomes a stepping-stone to dysplasia and cancer.
For example, colonic serrated polyps arise from differentiated cells through pyloric metaplasia, while conventional adenomas arise from stem cell dysregulation. Intriguingly, regenerative hijacking appears highly conserved across tissues: Barrett’s esophagus, pancreatic acinar-to-ductal metaplasia, and colon polyps share common molecular fingerprints.
The Immune System’s Silent Battle
Initially, cytotoxic T cells and NK cells vigilantly patrol tissues, eradicating precancerous clones through immunosurveillance. However, as aging and chronic inflammation set in, this dynamic shifts:
-
Cytotoxic immunity weakens.
-
Innate immune responses and inflammation dominate (“inflammaging”).
-
Lactate accumulation from metabolic reprogramming suppresses T cell and NK cell function.
Fibroblast senescence compounds the problem. Senescent cells release a toxic cocktail called the Senescence-Associated Secretory Phenotype (SASP) that suppresses immune clearance and promotes tumor growth. Thus, the transition from precancer to cancer often hinges not just on genetic changes in epithelial cells but on the collapse of immune defenses and stromal integrity.
Shaping the Future: Precision Prevention
Risk stratification must evolve. Instead of relying purely on histology or basic imaging, future screening should integrate:
-
Molecular clocks of biological aging
-
Somatic mutation burden profiling
-
Microenvironmental signatures like SASP factors or fibroblast senescence markers
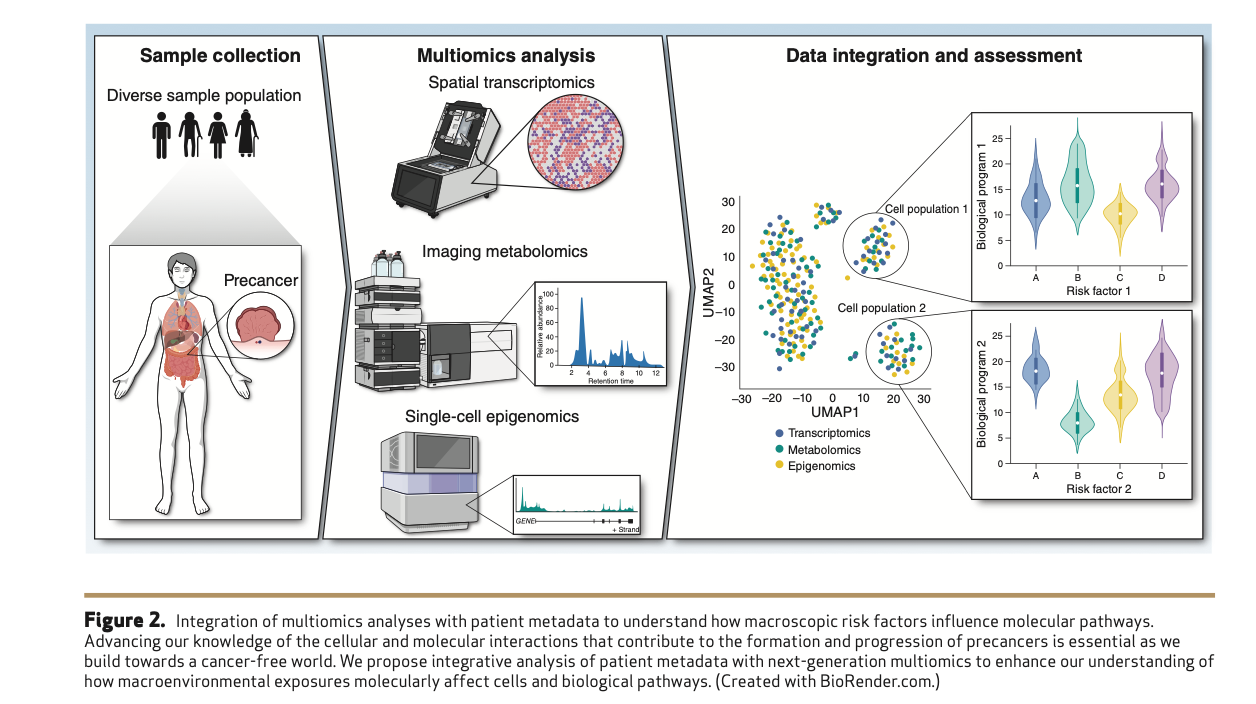
There’s a huge opportunity to apply immune-boosting interventions—potentially even immunotherapy—at the precancerous stage, where lesions are less genetically complex and more vulnerable. Additionally, there’s an urgent need for high-resolution, spatial, multi-omic atlases of precancer to guide both basic research and clinical decision-making. Public health strategies must also address macroenvironmental exposures: promoting anti-inflammatory diets, microbiome health, and reducing social disparities that accelerate biological aging.
Key Takeaways for Future Research
- Identifying which precancers will progress to avoid overdiagnosis/overtreatment
- Using cytotoxic immunity for controlling precancer
- Combining multi-omic and spatial information with patient metadata
- Investigating precursors to bladder and pancreatic cancers lacking effective prevention strategies
Understanding precancer biology has profound implications not just for screening and intervention strategies but also for public health policy development. The authors emphasize the multidimensional approach needed to advance our understanding of cancer development, bridging current knowledge gaps to potentially eliminate these devastating diseases. The holy grail of precancer research remains understanding which macroenvironmental factors influence cellular and molecular functions, predisposing specific cells to transformation and certain precancers to progress into malignancy.
You Can Read the Full Article on AACR Cancer Discovery
You Can Also Discover New Paper Alert Section on OncoDaily
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
