
South Korean Scientists at KAIST Reprogram Colon Cancer Cells in Breakthrough That Could Change Oncology
For decades, the cornerstone of cancer treatment has relied on strategies aimed at destroying malignant cells—primarily through chemotherapy, radiation, and immunotherapy. While these approaches have significantly improved survival rates for many cancers, they are often accompanied by serious drawbacks. Because these treatments do not distinguish perfectly between cancerous and healthy cells, collateral damage to normal tissues is common, leading to toxicity, immune suppression, fatigue, organ dysfunction, and a diminished quality of life. Moreover, some cancers develop resistance over time, making them more difficult to treat with conventional methods alone.
Amidst these challenges, a groundbreaking concept has emerged: cancer reversion. Unlike traditional therapies that aim to eliminate cancer cells, cancer reversion strategies seek to reprogram malignant cells into a benign, differentiated state, restoring their normal function and removing their ability to proliferate uncontrollably. This approach targets the underlying gene regulatory mechanisms that govern cell identity and fate.
In a recent pioneering study, scientists from the Korea Advanced Institute of Science and Technology (KAIST) demonstrated this concept in colon cancer. By identifying and inhibiting a set of key master regulatory genes—MYB, HDAC2, and FOXA2—they were able to reverse the malignant phenotype of colon cancer cells. These reprogrammed cells began to resemble normal enterocytes, the healthy epithelial cells of the intestinal lining, highlighting a promising shift in how we might treat cancer—not by destroying cells, but by restoring their original identity.
What Is Colon Cancer?
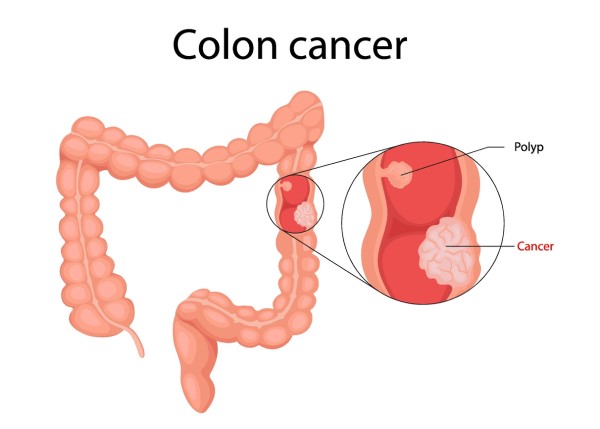
Colon cancer, also known as colorectal cancer when referring to both the colon and rectum, is a type of cancer that starts in the large intestine (colon), the final part of the digestive tract. Most colon cancers begin as small, benign growths called polyps, which can slowly turn cancerous over time. Screening methods like colonoscopy are crucial because they can detect and remove polyps before they become malignant.
Colon cancer is typically classified based on how far it has spread:
-
Localized (early-stage): confined to the inner wall of the colon.
-
Locally advanced: has spread to nearby lymph nodes or tissues.
-
Metastatic (advanced): has spread to distant organs like the liver or lungs.
Main Treatment Options for Colon Cancer
The treatment strategy for colon cancer depends on several factors, including the stage of the disease, the tumor’s molecular characteristics, and the patient’s overall health. In most cases, a multidisciplinary approach is used to tailor treatment plans and optimize outcomes. The core treatment modalities include surgery, chemotherapy, targeted therapy, immunotherapy, and, in select cases, radiation therapy.
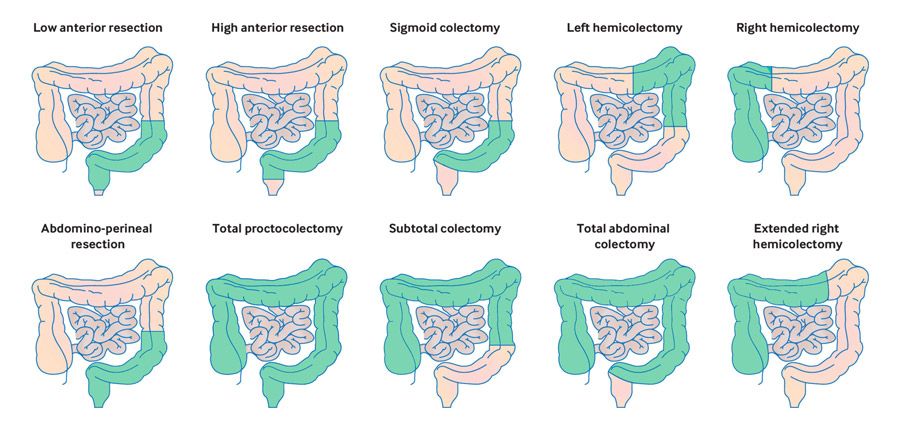
Surgery is typically the first-line treatment for early-stage colon cancer. In these cases, the primary goal is to remove the tumor along with a margin of healthy tissue and nearby lymph nodes through a procedure known as a colectomy. For patients with more advanced disease, surgery may still play a crucial role, often as part of a multimodal approach. In such cases, the operation is aimed at debulking the tumor, followed by adjuvant chemotherapy to eliminate residual cancer cells and reduce the risk of recurrence. Chemotherapy is commonly used both before and after surgery. When given postoperatively (adjuvant chemotherapy), its purpose is to destroy microscopic disease that may remain after surgical resection. In locally advanced or metastatic cases, chemotherapy can also be administered preoperatively (neoadjuvant chemotherapy) to shrink tumors and improve surgical outcomes. Two widely used chemotherapy regimens in colon cancer are FOLFOX (a combination of 5-fluorouracil, leucovorin, and oxaliplatin) and CAPOX (capecitabine with oxaliplatin).
Targeted therapy has become an important addition to the treatment landscape, particularly for patients with metastatic colon cancer. These therapies block specific signaling pathways that promote tumor growth. For example, EGFR inhibitors like cetuximab and panitumumab are effective in patients whose tumors are wild-type for RAS genes, while VEGF inhibitors such as bevacizumab are used to disrupt blood vessel formation in tumors. These agents are frequently administered in combination with chemotherapy to enhance response rates. Immunotherapy is reserved for a subset of colon cancers that exhibit microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR). These tumors are more likely to respond to immune checkpoint inhibitors, such as pembrolizumab and nivolumab, which activate the body’s immune system to recognize and destroy cancer cells. Immunotherapy has shown impressive results in metastatic settings and is being actively studied for use in early-stage disease. While radiation therapy is not routinely used in colon cancer, it may be considered in select advanced cases, particularly when tumors cause bleeding, obstruction, or pain. Radiation is more commonly applied in rectal cancer, but in colon cancer, it can provide palliative relief when surgery is not feasible.
Together, these treatment options form the foundation of modern colon cancer management, allowing for personalized and increasingly effective therapeutic strategies.
Understanding the Benefits and Side Effects of Colon Cancer Treatments
Every treatment for colon cancer brings important potential benefits—but also comes with risks and side effects that must be considered carefully. While the goal is always to eliminate the disease and preserve quality of life, patients may experience a range of physical effects depending on the treatment used. Understanding these possible side effects helps set realistic expectations and allows for early management.
- Surgery offers the best chance of cure in early-stage colon cancer but can be associated with pain, risk of infection, bleeding, and changes in bowel habits. Some patients may develop temporary or permanent digestive issues, such as diarrhea or constipation, depending on how much of the colon is removed.
- Chemotherapy helps reduce recurrence and control advanced disease but often causes fatigue, nausea, loss of appetite, and hair thinning. Specific drugs like oxaliplatin may lead to neuropathy—numbness or tingling in the hands and feet—which can sometimes be long-lasting.
- Targeted therapy, while often more focused than chemotherapy, is not without its effects. EGFR inhibitors may cause skin rashes, dry skin, and nail changes, while VEGF inhibitors like bevacizumab can increase the risk of high blood pressure, bleeding, and delayed wound healing.
- Immunotherapy, though generally better tolerated, can still lead to immune-related side effects. These may include inflammation of the skin, intestines, thyroid gland, or lungs, depending on which organs the immune system mistakenly targets. Most side effects are manageable if recognized early.
- Radiation therapy, though less common in colon cancer, can cause fatigue, abdominal cramping, and diarrhea. In rare cases, it may affect nearby organs or lead to bowel obstruction or strictures over time.
A New Hope for Colon Cancer: Reprogramming Tumor Cells Instead of Destroying Them
In a study published in Advanced Science (December 11, 2024), researchers from the Korea Advanced Institute of Science and Technology (KAIST), led by Professor Kwang-Hyun Cho, made a major discovery that could change how we treat colon cancer. Instead of trying to kill cancer cells, the team found a way to reprogram them into healthy, normal cells by turning off certain genes that keep them in a cancerous state.
The scientists focused on three key genes—MYB, HDAC2, and FOXA2—that act like “switches” helping cancer cells stay undifferentiated and grow uncontrollably.
- MYB is a gene that is often overactive in colon and blood cancers. It helps cancer cells grow and prevents them from maturing into normal cells.
- HDAC2 plays a role in tightly packing DNA, which can shut down important protective genes. This allows cancer cells to survive and keep multiplying.
- FOXA2 is usually involved in normal cell development, but in cancer, it can sometimes work the wrong way and help the tumor grow.
To find these key genes, the researchers used a powerful computer model called BENEIN, which analyzes the activity of thousands of genes in individual cells. This model helped them pinpoint MYB, HDAC2, and FOXA2 as the main drivers keeping the cancer cells in a harmful state. When the researchers turned off all three genes in colon cancer cells in the lab, the cancer cells began to slow their growth, lose their aggressive behavior, and start acting like healthy intestinal cells. This was a sign that the cells were “reverting”—going back to a more normal, non-cancerous state.
To confirm their results, they tested this method in mice. The mice that received the reprogrammed cancer cells developed much smaller tumors compared to those that received untreated cancer cells. When the scientists studied the gene activity in these cells, they found that the reprogrammed cells looked very similar to normal colon tissue, showing that the therapy had successfully transformed cancerous cells into healthier ones. This discovery suggests that instead of attacking cancer cells with toxic drugs, we may one day be able to “teach” cancer cells how to behave normally again—a potentially safer and more effective way to treat certain cancers.
What Is Cancer Reversion? How Scientists Are Reprogramming Tumor Cells to Become Healthy Again
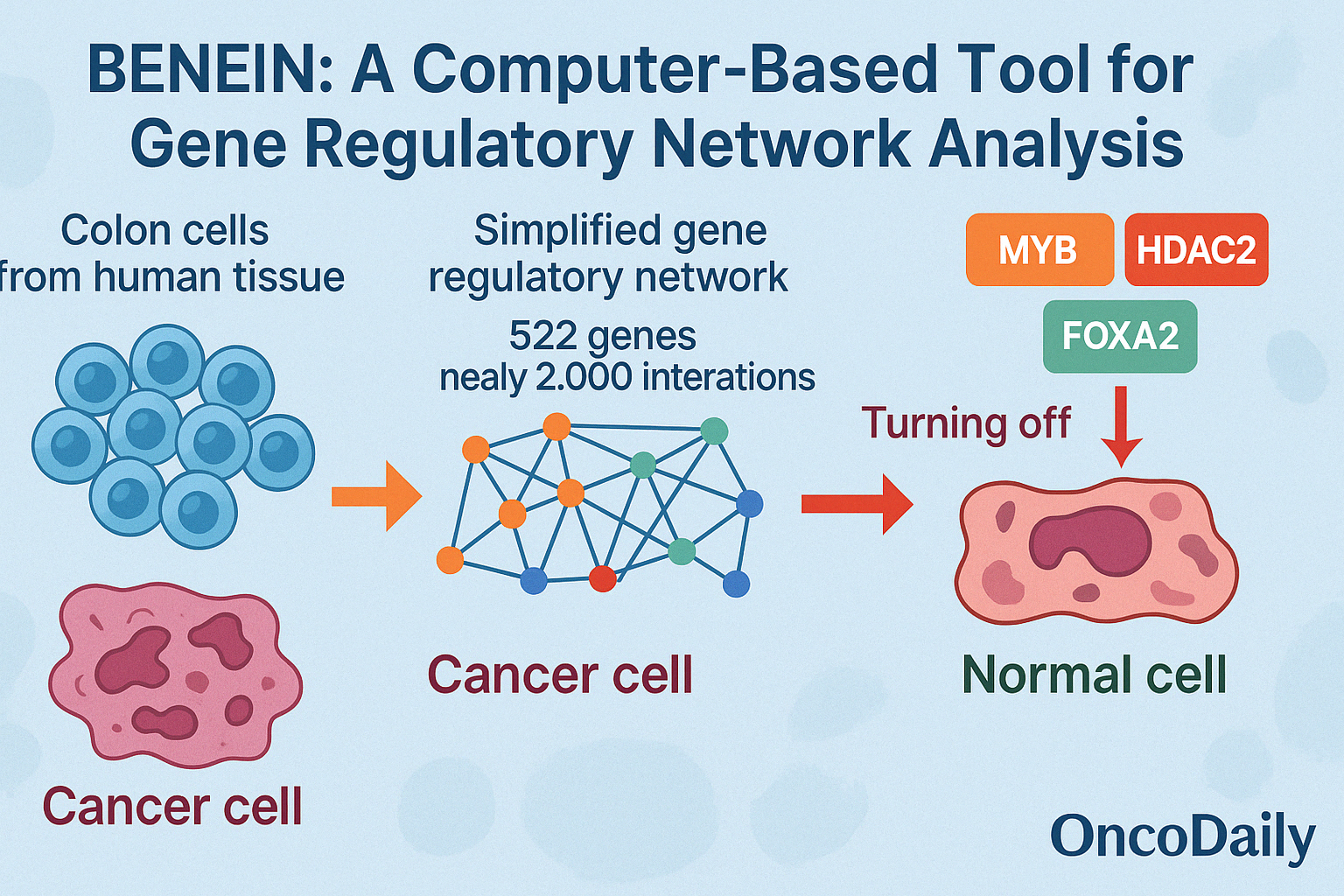
In their groundbreaking research, Gong and colleagues (2024) introduced a powerful computer-based tool called BENEIN (short for Boolean Network Inference and Control). This tool was designed to understand how genes work together inside a cell and how they influence a cell’s identity—whether it stays as a cancer cell or becomes a healthy one. To do this, the researchers studied thousands of colon cells from human tissue, looking at which genes were turned “on” or “off” in each cell. BENEIN helped them build a detailed map of how genes interact, like a network where each gene is a light switch, and each connection controls whether other switches turn on or off. This kind of model allows scientists to simulate what might happen if certain key genes are blocked or activated.
In this case, the team focused on 4,252 cells that were in the process of turning into normal intestinal cells called enterocytes. They created a simplified network involving 522 genes and nearly 2,000 interactions, eventually narrowing it down to a core group of 13 key regulators. By running simulations on this network, the scientists discovered something exciting: if they turned off three specific genes—MYB, HDAC2, and FOXA2—the model predicted that the cancer cells would shift away from a malignant state and begin behaving like healthy cells. These stable gene patterns, known as attractors, represent the “fate” of the cell. The simulation showed that blocking these three genes was enough to push the cell from a cancerous fate to a normal one—like flipping the right switches to change the course of the disease.
What makes this breakthrough even more exciting is that it was done without permanently altering the genes (no gene editing)—just by identifying and controlling the natural regulatory signals that cells use every day. This makes BENEIN a powerful tool not just for colon cancer research, but for understanding how to safely guide cells back to health in many types of diseases.
A Gentler Way to Treat Cancer by Reprogramming Cells
This research represents a major shift in how we think about treating cancer. Instead of using powerful drugs to destroy cancer cells—often harming healthy cells in the process—scientists are exploring the idea of “precision reversion therapy.” This means using advanced tools to identify the unique gene activity in a person’s tumor, then gently guiding the cancer cells to stop acting aggressively and behave more like normal cells.
By focusing on the specific gene networks that drive a person’s cancer, doctors could one day design personalized treatments that quietly reprogram the tumor instead of attacking it. These therapies might reduce the risk of the cancer coming back or becoming resistant to treatment. One of the most exciting possibilities is that this approach could reduce the harsh side effects that often come with traditional cancer treatments like chemotherapy and radiation. Because reversion therapy doesn’t kill cells but changes how they behave, it could protect healthy tissues and preserve the immune system. This would be especially helpful for older adults or people too weak to handle strong medications.
However, there are still challenges ahead. Not all cancer cells are the same, even within one tumor, which makes it harder to reprogram them all at once. And scientists are still figuring out how best to deliver these gene-modifying toolsinto solid tumors safely and effectively—options being studied include tiny nanoparticles or targeted gene delivery systems. More testing in different types of cancers and living organisms is also needed to make sure this approach is safe and long-lasting. Still, this research signals a powerful change in cancer care. It suggests that instead of waging war on cancer, we might learn to “re-educate” cancer cells, helping them return to a peaceful, healthy state. Thanks to advances in computational biology, single-cell analysis, and gene therapy, this idea of programmable cancer reversion is moving closer to real-world treatment—and with it, a future of cancer care that is smarter, gentler, and more personal than ever before.
Turning Off Cancer Genes—Without Editing DNA
One of the most promising aspects of this new cancer reprogramming approach is that it doesn’t require permanent genetic editing. Instead, researchers are exploring non-invasive and reversible ways to “turn off” the genes that help cancer cells stay dangerous.
Take MYB, for example—a gene that is often overactive in colon and blood cancers. Because of its structure, it’s been tough to target with traditional drugs. But scientists have found newer tools like RNA interference (siRNA), antisense oligonucleotides (ASOs), and CRISPR interference (CRISPRi) that can block MYB at the message level, stopping it before it makes harmful proteins. Even some natural compounds, like Celastrol, have shown early promise in reducing MYB activity in lab studies. On the other hand, HDAC2—another cancer-driving gene—is already being targeted in patients. HDAC2 helps cancer cells by shutting down important protective genes. But doctors can use a group of medicines called HDAC inhibitors(HDACi), such as vorinostat, romidepsin, and panobinostat, which are already approved or being tested for cancer treatment. These drugs help loosen tightly packed DNA, allowing normal, healthy genes to work again and encouraging cancer cells to return to a more normal state.
FOXA2, the third key gene, is a bit more complex. It plays different roles in different tissues—sometimes helping, sometimes harming. While there are no direct drugs for FOXA2 yet, researchers can still reduce its effects by blocking the signals that activate it, or by using gene-silencing tools like RNAi or CRISPRi. Because of FOXA2’s mixed roles, it’s important to personalize this approach for each patient. Delivering these treatments directly to tumors is also becoming easier. New technologies like lipid nanoparticles (LNPs), tumor-targeted viruses, and even custom-made exosomes are making it possible to safely deliver gene-silencing tools to the right cells, with fewer side effects.
Altogether, these advances offer a powerful set of tools to support cancer reversion therapy. Rather than permanently changing DNA, they allow temporary and controllable silencing of harmful genes. This matches the flexible, reversible nature of the cell state changes modeled by the BENEIN system. With further development, turning off genes like MYB, HDAC2, and FOXA2 may soon become a standard, low-impact way to treat cancer—by helping cells remember how to be normal again.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What is colon cancer?
Colon cancer is a type of cancer that begins in the large intestine (colon). It usually starts from benign polyps that can become cancerous over time. Early detection through screening is key to prevention and treatment.
How is colon cancer usually treated?
Treatment options include surgery, chemotherapy, targeted therapy, immunotherapy, and sometimes radiation therapy. The approach depends on the cancer stage, molecular profile, and the patient’s overall health.
What is cancer reversion therapy?
Cancer reversion therapy is a novel treatment approach that aims to reprogram cancer cells into normal, non-cancerous cells instead of killing them. It targets the genetic regulatory mechanisms that define a cancer cell’s identity.
What are MYB, HDAC2, and FOXA2, and why are they important?
These are master regulatory genes involved in keeping colon cancer cells in a malignant state. Inhibiting them helps reprogram the cells back into healthy intestinal cells, as demonstrated in recent KAIST research.
What is BENEIN, and how does it work?
BENEIN (Boolean Network Inference and Control) is a computational model that maps gene interactions in cells. It identifies key genes controlling cell identity and helps simulate how turning off certain genes could revert cancer cells to normal.
Is cancer reversion therapy already available for patients?
Not yet. While early studies in lab settings and mice have shown promise, this therapy is still in preclinical stages and requires further research and clinical trials before becoming a standard treatment.
Does cancer reversion involve gene editing?
No. This approach uses non-permanent, reversible techniques like RNA interference (siRNA), antisense oligonucleotides (ASOs), and CRISPR interference (CRISPRi) to turn off cancer-promoting genes without altering DNA permanently.
Can reversion therapy reduce the side effects of cancer treatment?
Yes, potentially. Since this therapy doesn’t destroy cells but reprograms them, it could reduce collateral damage to healthy tissues and minimize side effects compared to chemotherapy or radiation.
Will reversion therapy work for all cancers?
Probably not all. Tumor types differ greatly, and not all have the same gene regulation mechanisms. However, reversion therapy could be highly effective for specific cancers, especially those with known gene drivers like MYB or HDAC2.
What is the future outlook for cancer reversion therapy?
The future is promising. As tools like BENEIN and gene-silencing technologies improve, personalized, low-toxicity cancer treatments may become more common. Clinical trials will be key to determining real-world safety and effectiveness.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
