
Steve Harvey: 30 ADC-related clinical trials this year
Steve Harvey, CEO of Camena Bioscience, shared a post on LinkedIn:
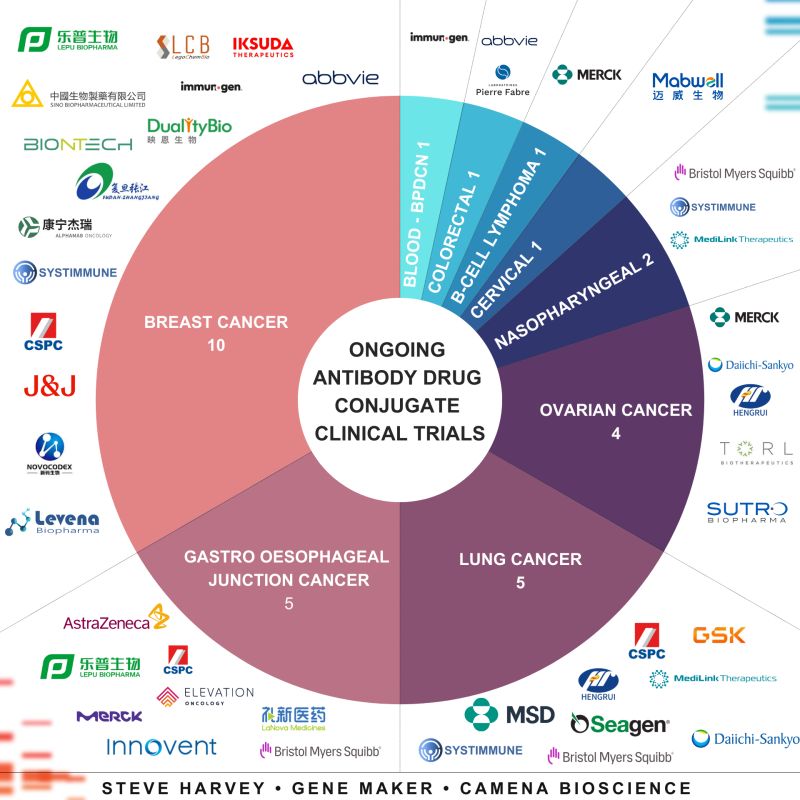
“Antibody-drug conjugates (ADCs) are a great weapon against cancer. There are 30 ADC-related clinical trials this year.
The infographic shows the cancers those ADCs target and the companies developing or licensing those assets.
Here are the phases they’re in:
– Phase 2 = 2
– Phase 2/3 = 3
– Phase 3 pending = 5
– Phase 3 = 20
ADCs work by targeting a specific cancer cell type (that’s the antibody bit). Linked to that antibody is a drug that kills the cancer cell.
Broadly, these trials are using two types of drugs:
Tubulin Inhibitors:
Tubulin performs a broad range of cellular functions, including cell division. Inhibiting tubulin leads to cell death (apoptosis).
Topoisomerase Inhibitors:
Topoisomerases unwind DNA during replication/division, transcription and repair. If a cell can’t carry out these functions, it causes cell death (apoptosis).
Another neat trick of ADCs is the linker between the antibody and drug.
The ADCs in these trials use a range of linkers, but the design principles are similar. You want the drug to be released when the antibody finds a specific cancer cell.
For example, one trial uses a valine-vitrulline linker. That short amino acid sequence is cut by an enzyme (cathepsin B), which is highly expressed in many tumour cells.
As the ADC moves through our blood, it remains stable, but the drug is released when it finds the cancer.
While Camena Bioscience is a DNA synthesis company, pharma companies use our gSynth technology to discover antibodies.
Feel free to repost this if you found it helpful.”
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
