Richard Possemato, Associate Professor of NYU School of Medicine, shared on X:
“I’m excited to share new work out of the lab in Nature Metabolism today on the impact of tumor-relevant glucose concentrations on the response to pyrimidine synthesis inhibitors.
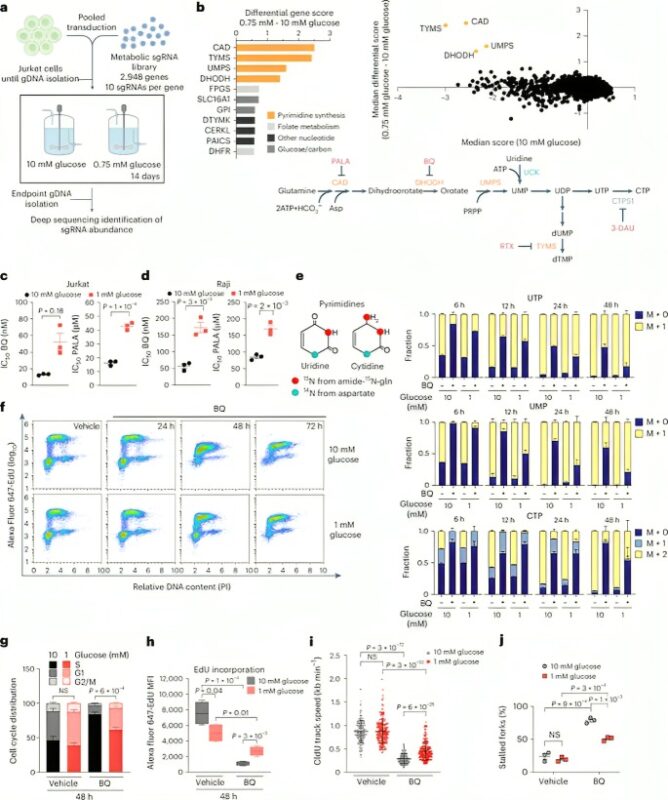
In this study postdoc Minwoo Nam used a continuous flow cell culture system that we developed over 10 years ago with Kivanc Birsoy to maintain cells at tumor-relevant concentrations of glucose (1 mM) and perform CRISPR-based genetic screening.
Remarkably, Minwoo found that targeting de novo pyrimidine synthesis, but not other metabolic pathways, was much more effective in high (10mM) glucose conditions. Cells in high glucose halted replication faster and underwent apoptosis more rapidly.
Minwoo found that in high glucose, UDP-glucose synthesis was maintained at a much higher level, shunting pyrimidines away from DNA and RNA synthesis. Blocking nucleotide sugar synthesis permitted cells to maintain replication longer when pyrimidines were limiting.
Blocking pyrimidine synthesis potently triggered apoptosis even when we targeted steps after the UDP-glucose shunt. Culture in low glucose potently suppressed apoptosis induced by inhibiting pyrimidine synthesis, indicating a second effect of glucose on apoptosis signaling.
Deleting BAK or Caspase 9 could completely block apoptosis induced by pyrimidine synthesis inhibitors. Moreover, Bcl2 inhibitors or death receptor-dependent methods of inducing apoptosis were not affected by glucose levels.
Thus, Minwoo concluded that glucose level impacts a nuclear to mitochondrial signal that triggers intrinsic apoptosis in these p53-null cancer cells. Future work in the lab will hopefully uncover that unknown mechanism!
Because many of the enzymes in de novo pyrimidine synthesis are current and proposed anti-cancer targets and the glucose level in tumors is typically low, this work might indicate a key mode of failure for such treatments.
Upon inhibition of replication in cancer cells, activation of the ATR/CHK1 pathway helps maintain viability. ATR/CHK1 inhibition cooperates strongly with pyrimidine synthesis inhibitors. However, current ATR and CHK1 inhibitors are not well-tolerated.
We’re grateful to our collaborators Jessica Spinelli at UMASS Worcester and Tony Huang, and support from Perlmutter Cancer Center at NYU Langone Health and NYU GSOM Pathology. Come find us at possematolab.org for more on our work!”
Authors: Minwoo Nam, Wenxin Xia, Abdul Hannan Mir, Alexandra Jerrett, Jessica B. Spinelli, Tony T. Huang, Richard Possemato

