
Roger Li: Final results from CORE2 Trial
Roger Li, Urologic Oncologist at Moffit Cancer Center, shared on X:
“Out now Nature Medicine.
Final results from CORE2 combining IVe Creto+Nivo in cis-ineligible MIBC patients.
No DLTs
pCR = 42.1%
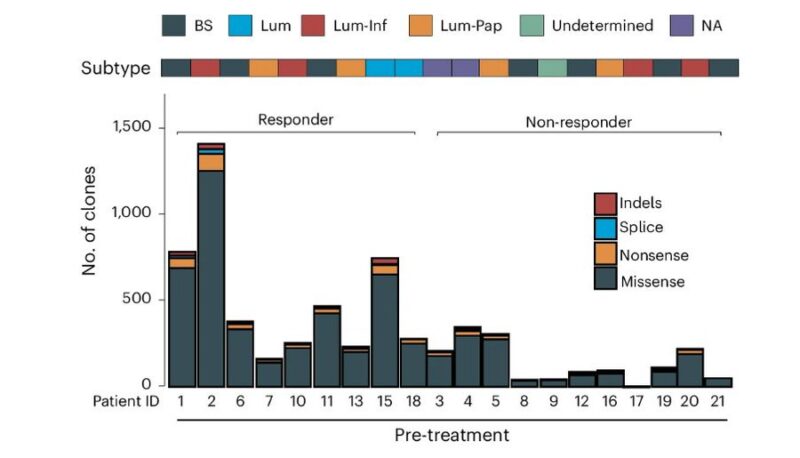
pCR assoc with baseline free E2F activity, TMB
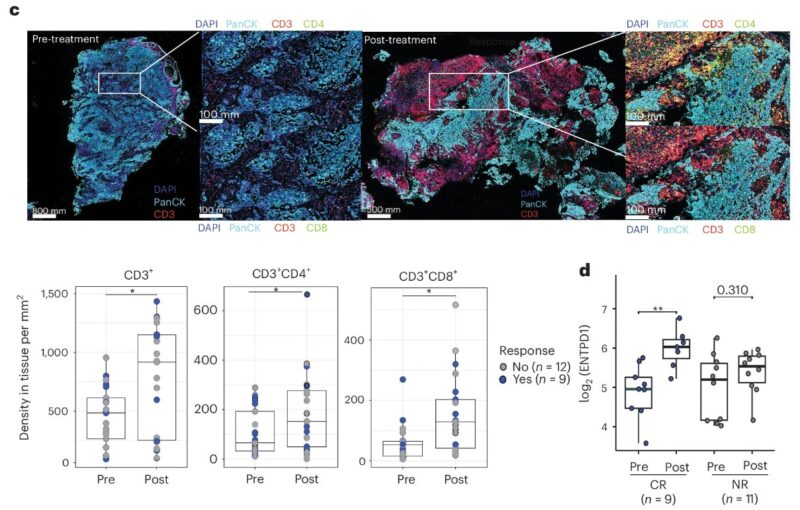
Increased T cell infiltration
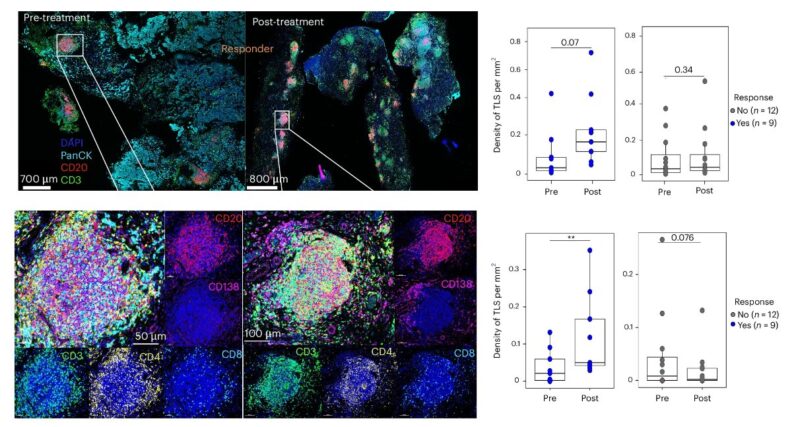
Formation of TLS assoc with pCR.
Out of 21 patients enrolled, 20 completed treatment, 17 undwerent radical cystectomy.
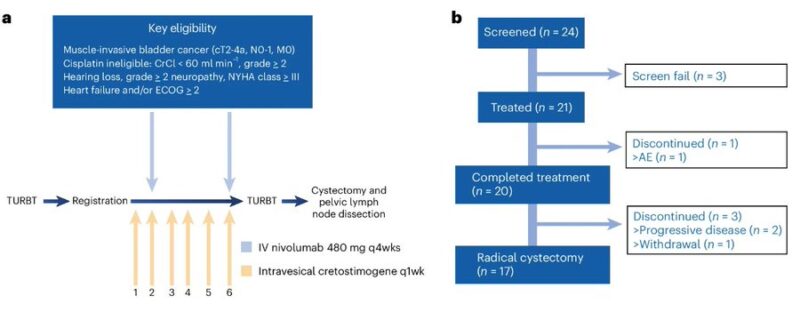
8/19 evaluable patients with pCR (pT0N0), 1 year RFS 70.4%.
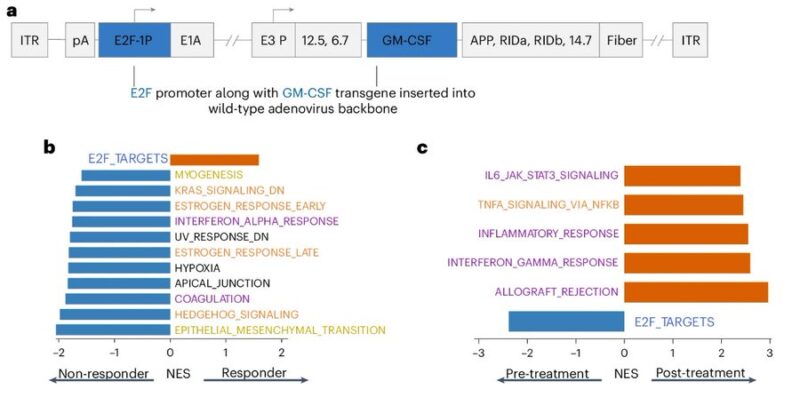
Baseline expression of E2F targets was higher in responders, corroborating MOA of Creto, engineered with hE2F1 promoter. In non-responders, E2F targets on post-treatment tumors were lower, suggesting E2F expressive tumor clones eliminated by treatment.
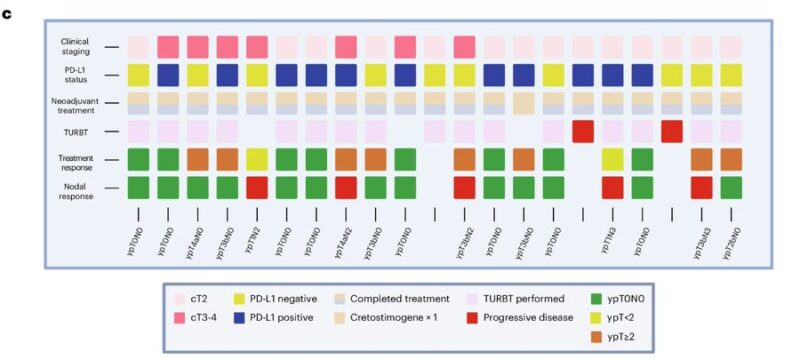
pCR was associated with baseline TMB but not PD-L1 level, suggesting Creto turning cold into hold TiME.
We found increased T cell infiltration post-treatment, correlated with increased urinary T-cell attracting chemokines following treatment.
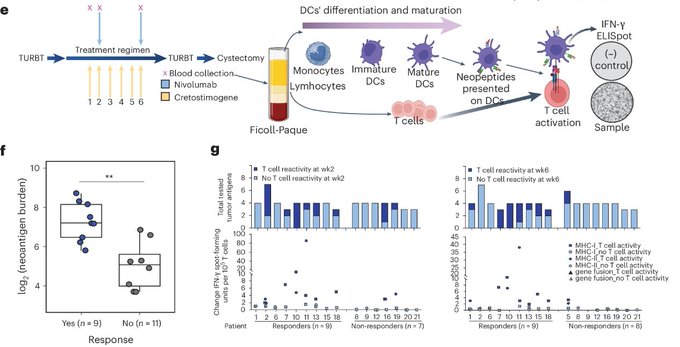
We predicted neoantigens from WES/RNAseq data from baseline tumors, and tested reactivity against these neopeptides using PBMCs collected along the treatment course. Neopeptide specific T cell reactivity was higher in pts with pCR.
Most intriguingly, TLS were found to be induced ONLY in pts with pCR, suggesting importance of anti-tumor humoral immunity, along with increased levels of urinary B-cell attracting chemokine CXCL13.
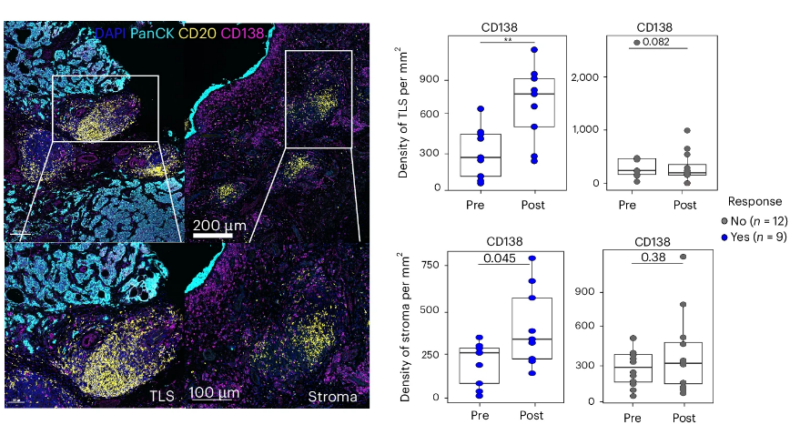
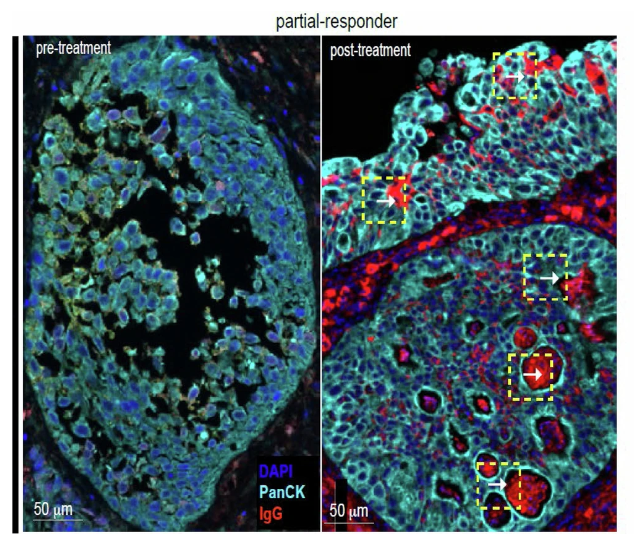
There was also evidence of maturation of TLS, as more CD138+ plasma cells found within TLS post-treatment, along with isotype switching from IgM – IgG/A.
In 1 patient with downstaging to CIS on post-treatment TUR, there was evidence of IgG coating of tumor cells by IgG, suggesting tumor specific humoral response.
Second article this year published in Nature Medicine this year on results from combination trials using IVe Creto + ICB. Important insights uncovered herein will certainly drive future trials.
It’s been an amazing journey x4 yrs since trial opened. Thank you to all of my co-investigators, especially my mentors Jose Conejo-Garcia and James Mule.
Thanks to CG oncology for the sponsorship. Thanks to Department of Defense, Bladder Cancer Advocacy Network, Moffitt Cancer Center for funding support.
Kuan Arthur and James Burke: this trial could never have happened without your support, Thank you!”
Oncolytic immunotherapy with nivolumab in muscle-invasive bladder cancer: a phase 1b trial
Authors: Roger Li, Nancy Y. Villa, Xiaoqing Yu, Joseph O. Johnson, Gustavo Borjas, Jasreman Dhillon, Carlos M. Moran-Segura, Youngchul Kim, Natasha Francis, Denise Dorman, John J. Powers, Wade J. Sexton, Philippe E. Spiess, Michael A. Poch, Logan Zemp, Scott M. Gilbert, Jingsong Zhang, Julio M. Pow-Sang, Alexander R. A. Anderson, Tingyi Li, Xuefeng Wang, G. Daniel Grass, James M. Burke, Colin P. N. Dinney, Paulo C. Rodriguez, Rohit K. Jain, James J. Mulé, Jose R. Conejo-Garcia

More posts featuring Roger Li on oncodaily.com
Dr. Roger Li is a urologic oncologist at Moffitt Cancer Center, specializing in the surgical treatment of bladder, prostate, kidney, and penile cancers. He offers open, laparoscopic, and robotic-assisted procedures, including nerve-sparing options. Dr. Li received advanced training in urologic oncology at MD Anderson Cancer Center. Dr. Li researches personalized therapies through genomic profiling and develops new immunotherapies for early-stage bladder cancer.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
