Benjamin Auberger: This major study naturally raises several questions
Quoting Benjamin Auberger, Oncologist at CHRU Brest, on LinkedIn:
“A wow presidential session with a profound change in our practices in metastatic urothelial carcinoma!
Our goal as medical oncologists is to improve the outcome and when we succeed so dramatically in one of the most aggressive cancers (200,000 deaths/year in the world), it’s truly a profound moment!
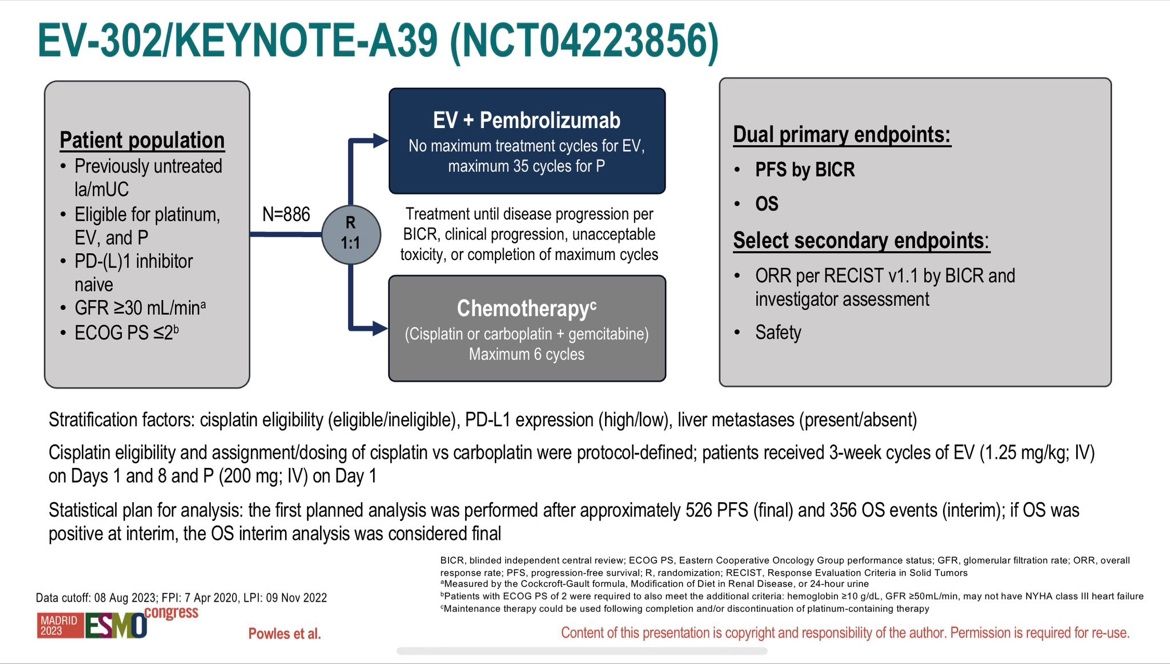
Today, superstar Tom Powles reported the long-awaited results of the phase 3 study (EV-302) focusing on the effectiveness and tolerance of a combination with Enfortumab Vedotin (an antibody-drug conjugate (ADC) validated in 3rd line metastatic) and Pembrolizumab (anti-PD1) compared to standard treatment for more than 30 years with platinum-containing chemotherapy (carboplatin/cisplatin) and gemcitabine in patients with metastatic urothelial carcinoma in 1st line setting (n=886)!
After a median follow-up of 17.2 months, the data are spectacularly in favor of the combination (n=442):
- 55% decrease risk of disease progression with a median Progression-free survival (PFS)
of 12.5 months vs 6.3 months (HR=0.45; 95% CI: 0.38-0.54; p<0.00001)! - Overall survival almost doubled with a median Overall Survival (OS) of 31.5 months vs 16.1 months (HR=0.47; 95% CI: 0.38-0.58; p<0.00001)!
- Superiority is confirmed regardless of the cisplatin eligibility and the PD-L1 status!
Objective response rate ORR of 67.7% vs 44.4% (p<0.00001) with 29% of complete response!
No new safety signal against the association with grade ≥3 treatments-related adverse event occurred in 55.9% with Enfortumab and Pembrolizumab and 69.5% with chemotherapy. Particular attention concerning skin toxicity (15.5% skin reactions with Enfortumab and 11.8% with Pembrolizumab) and the occurrence of peripheral neuropathy (6.8%) or hyperglycemia (6.1%)
This major study naturally raises several questions:
- How to extrapolate these data in a first-line situation, knowing that the therapeutic standard is currently platinum-containing chemotherapy with gemcitabine (4 to 6 cycles) followed by maintenance treatment with Avelumab, in stable patients or in therapeutic response after chemotherapy ?
- What will be therapeutic sequence in the 2nd line situation (chemotherapy? Erdafitinib in patients with select fibroblast growth factor receptor alterations?)
- Where will Sacituzumab and others ADCs be positioned? in 1st line with another immunotherapy (TROPHY-U01)? A triplet with Enfortumab and Pembrolizumab? In 2nd line in un-responding patients or with intolerance?
- How responsive will our competent authorities be in order to quickly offer our patients this considerable advance (another early access platform?)
Returning home with these new therapeutic standards to come fills us with joy and hope for our patients! Cancer fighters rock!”
Source: Benjamin Auberger/LinkedIn
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
