
Steven O’Day: At the Forefront of Immunotherapy
Dr. Steven O’Day, an immunotherapy pioneer and medical oncologist, is the Chief Medical Officer at Agenus, a leading innovator in the field of immuno-oncology.
Joining Agenus in 2020, Dr. O’Day is responsible for spearheading the company’s expansion in the immuno-oncology field.

With a distinguished background as a medical oncologist, Dr. O’Day has played a pivotal role in advancing cancer therapies for over three decades, particularly those targeting CTLA-4 inhibition.
His contributions to immunotherapy were integral to the development of groundbreaking treatments like Yervoy (ipilimumab) and Opdivo (nivolumab), which have transformed the landscape of cancer care.
Notably, he played a key role in treating the first patients with ipilimumab, the first checkpoint antibody (anti-CTLA-4). His presentation of seminal data at the ASCO plenary session in 2010 showcased the curative potential of anti-CTLA-4 and its long-lasting survival benefits in patients with metastatic melanoma. This pivotal research directly contributed to the FDA approval of ipilimumab in 2011.
About Agenus
Agenus Inc. is a clinical-stage immuno-oncology company focused on the discovery and development of therapies that engage the body’s immune system to fight cancer. Founded in 1994 and headquartered in Lexington, Massachusetts, Agenus has built a diverse pipeline of immune-modulating antibodies, cancer vaccines, and adoptive cell therapies.
The company’s approach to cancer immunotherapy is multifaceted, encompassing checkpoint inhibitors, bispecific antibodies, cell therapies, cancer vaccines, and adjuvants. Botensilimab, the company’s lead candidate, exemplifies its commitment to developing innovative immunotherapies to address unmet medical needs in oncology.
“Agenus is the most innovative company in this field with platforms and capabilities that have produced an outstanding pipeline of clinical and preclinical agents. I am excited to join Agenus’ pathbreaking scientific and clinical team. We share a bold vision for the future,” said Dr. Steven O’Day. “Together, we will accelerate optimal combination treatments for patients with cancer. This is a transformative period in the company’s history, and I am thrilled to join the team.”
Find more about Agenus in the latest article by OncoDaily
Mapping a Journey
Prior to his role at Agenus, Dr. O’Day was the Executive Director of the John Wayne Cancer Institute and Cancer Clinics at Providence St. John’s Health Center. He also led the Melanoma and Cutaneous Oncology Research Center there.
After receiving his BA in chemistry from Williams College, Dr. O’Day was chosen as a visiting scholar in philosophy and medical ethics at Oxford University. He earned his MD and completed his Internal Medicine Residency at Johns Hopkins University School of Medicine, followed by a Medical Oncology and Bone Marrow Transplant Fellowship at the Dana-Farber/Harvard Cancer Center.
Recognized as a leader in melanoma and immunotherapy, Dr. O’Day has significantly influenced drug development through his extensive involvement as a principal investigator in over 200 clinical trials, including many large international Phase III studies.
Dr. O’Day has served as a principal investigator for over 200 clinical trials, including key international pivotal trials where he has taken on leadership roles.
Where are we with BOT/BAL?
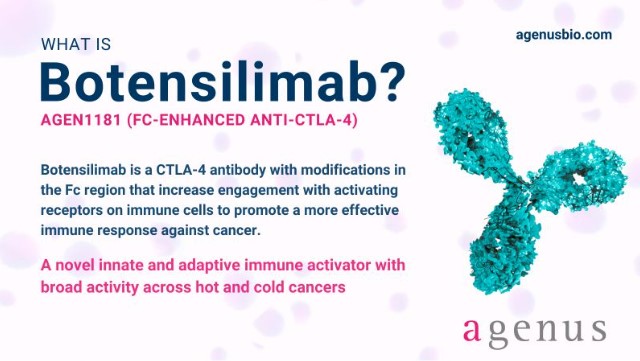
Botensilimab (BOT) is an investigational human Fc-enhanced, multifunctional anti-CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) antibody being developed by Agenus Inc. for the treatment of various solid tumors.
As a next-generation CTLA-4 inhibitor, botensilimab represents a promising new approach to enhancing anti-tumor immune responses, particularly in “cold” tumors that have historically been resistant to immunotherapy.
The first-in-human study of botensilimab (NCT03860272) was a phase 1/2 dose-escalation and expansion trial in patients with advanced solid tumors. It included cohorts with microsatellite stable metastatic colorectal cancer (MSS mCRC), a tumor type resistant to current immunotherapies.
The potential of botensilimab, along side with balstilimab (BAL), an investigational PD-1 antibody, is also being explored in the neoadjuvant setting through the NEST-1 trial (NCT05571293) in resectable colorectal cancer.
Additionally, the combination of BOT/BAL has also shown clinical activity in patients with refractory sarcomas, a population with limited treatment options during a Phase 1 clinical trial. The results were demonstrated during ESMO 2024.
“The updated sarcoma data presented at ESMO underscore the transformative potential of botensilimab and balstilimab for patients with refractory sarcomas who have exhausted other treatment options,” said Dr. Steven O’Day. “Seeing these metastatic sarcoma patients experience tumor reduction, with significant and durable responses is incredibly encouraging. BOT/BAL not only offers hope to patients with sarcoma but also holds promise for redefining the standard of care across other historically IO-resistant cancers.”
The encouraging efficacy data, coupled with a manageable safety profile, positions botensilimab as a potential game-changer in oncology. It is one of the drugs featured in OncoDaily’s “10 Most Promising Cancer Drugs Not Yet Approved: Solid Tumors – 2024 edition by OncoDaily.”
In this short video, Dr. Steven O’Day discusses botensilimab and its innovative mechanism of action in targeting immune checkpoints to enhance anti-tumor responses.
Beyond his research contributions, Dr. O’Day has held prominent positions within professional organizations. He has served on the ASCO Education Committee, focusing on melanoma, and has been active in the ASCO Communications Committee. He is dedicated to mentoring the next generation of oncologists, notably through the ASCO International Development and Education Award (IDEA) Working Group.
Additionally, he shares his insights as the host of the podcast “The Immuno-Oncology Curve,” further disseminating knowledge in the field.
In conclusion, Dr. O’Day’s leadership and vision not only have paved the way for groundbreaking advancements in cancer treatment but also continue to inspire future generations in the field. His unwavering commitment to innovation and patient care remains a guiding force in the ongoing fight against cancer.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
