Carey Jansen, Internal Medicine Resident at Yale-New Haven Hospital, shared a post on X:
“Our publication on pre-op SRS and the immune microenvironment of human brain metastases (BrM) is finally online at Nature Communications! This one was a journey from inception to publication, so we’re excited to share. See below for highlights!
Authors: Caroline S. Jansen, Meghana S. Pagadala, Maria A. Cardenas, Roshan S. Prabhu, Subir Goyal, Chengjing Zhou, Prasanthi Chappa, BaoHan T. Vo, Chengyu Ye, Benjamin Hopkins, Jim Zhong, Adam Klie, Taylor Daniels, Maedot Admassu, India Green, Neil T. Pfister, Stewart G. Neill, Jeffrey M. Switchenko, Nataliya Prokhnevska, Kimberly B. Hoang, Mylin A. Torres, Suzanna Logan, Jeffrey J. Olson, Edjah K. Nduom, Luke del Balzo, Kirtesh Patel, Stuart H. Burri, Anthony L. Asher, Scott Wilkinson, Ross Lake, Aparna H. Kesarwala, Kristin A. Higgins, Pretesh Patel, Vishal Dhere, Adam G. Sowalsky, Hannah Carter, Mohammad K. Khan, Haydn Kissick, Zachary S. Buchwald.

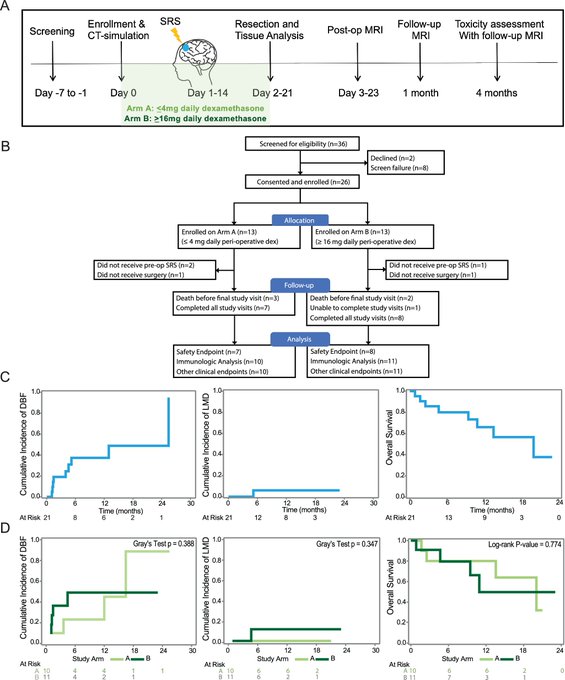
SRS + surgery, used for managing BrM, is usually given after surgery. Here, our trial with pre-op SRS evaluated clinical outcomes and impact on the immune response in BrM, finding that pre-op SRS could be delivered safely with similar efficacy to post-op SRS.
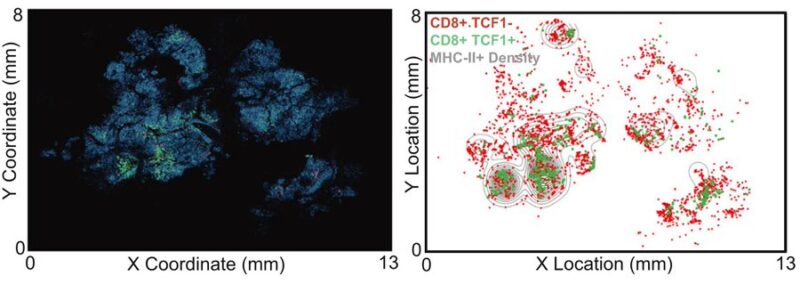
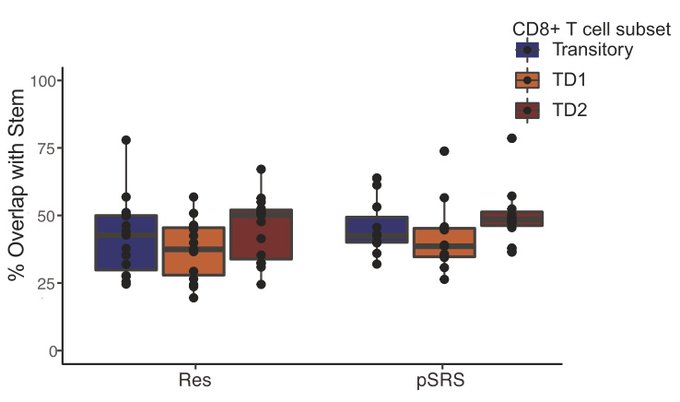
We found that a stem-like T cell subset is organized into immune niches in BrM, and the density of these immune niches is associated with local BrM recurrence.
After exposure to radiation via pre-op SRS, total CD8 T cell density was reduced, but importantly, immune niches were maintained regardless of radiation exposure, and dose of dexamethasone did not impact the immune microenvironment in BrM.
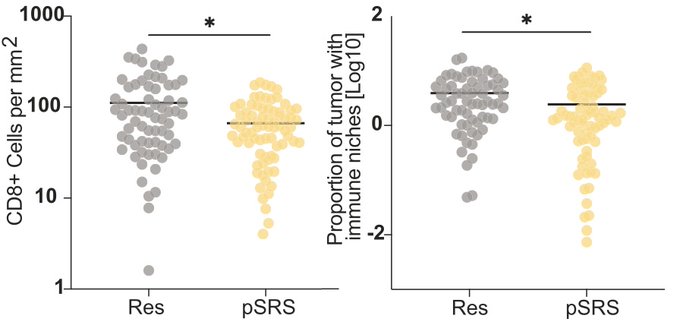
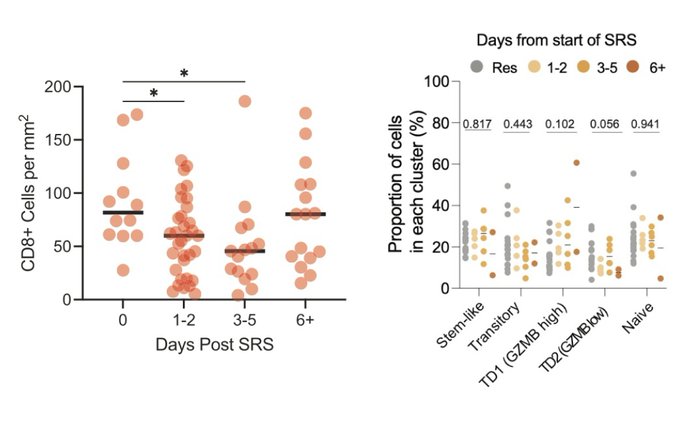
Interestingly, we found that the CD8 T cell response rebounded at 6+ days following SRS, and with an increased frequency of terminal effector-like cells, which may have implications for optimizing the sequencing or combination of multi modes of therapy (e.g. SRS + IO).
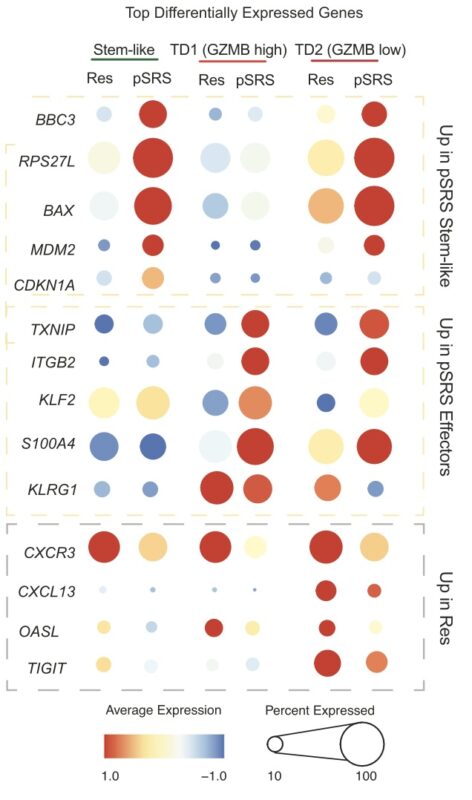
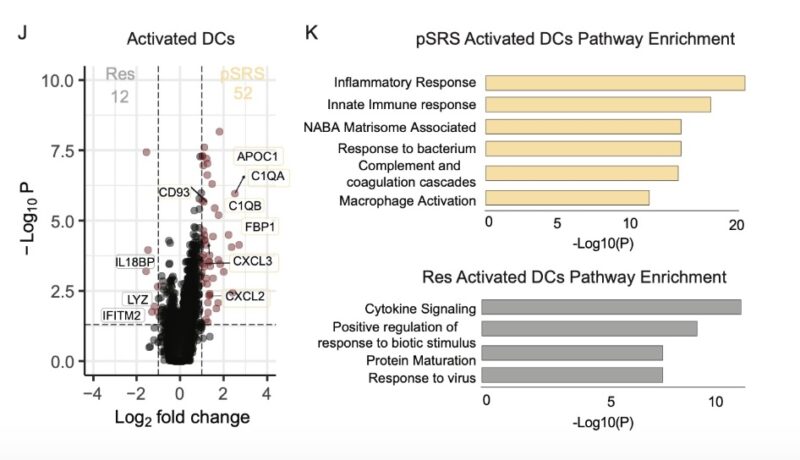
Additionally, by transcriptional analysis, we observed that pre-op SRS induced an inflammatory phenotype in BrM dendritic cells and promoted the accumulation of terminal effector cells expressing high levels of GzmB.
By TCR clonotype analysis, we demonstrate that these terminal effector cells are descendants of the BrM stem-like T cells, in both the surgery alone and pre-op SRS groups.
Together these data demonstrate an effective anti-tumor immune response can be mounted in BrM despite the CNS’ status as ‘immune privileged/restricted’ and that pre-op SRS can modulate this response with a decrease and subsequent rebound in CD8 T cells 6+ days following SRS.
This rebound is driven by an increased frequency of terminal effectors, likely due to differentiation of the in-situ BrM stem-like T cell population.
In sum, our data demonstrates that pre-op SRS is safe and therapeutically beneficial, and, importantly, these data provide a framework for how SRS may be leveraged to maximize intracranial anti-tumor CD8 T cell response.
This was a fun collaboration between Haydn Kissick and Zach Buchwaldlab, as well as many others. I am grateful to each of you, and to