Pashtoon Kasi: Here’s a cool precision medicine story about Liquid Biopsies
Quoting Pashtoon Kasi, oncologist and researcher at Weill Cornell Medicine on X/Twitter:
“Here’s a cool precision medicine story about Liquid Biopsies. We did a webinar with the Cholangiocarcinoma Foundation. A patient’s family from Australia reached out inquiring if I could take a look at their tumour’s Next-generation sequencing (NGS) report. 70 gene testing only showed TP53. I call this a “bland” report.
A “bland” report that doesn’t have anything actionable, positive or negative, needs to be investigated further. A more comprehensive test with DNA and RNA is of value to make sure we aren’t missing any fusions. Important for cholangiocarcinoma – a target-rich disease.
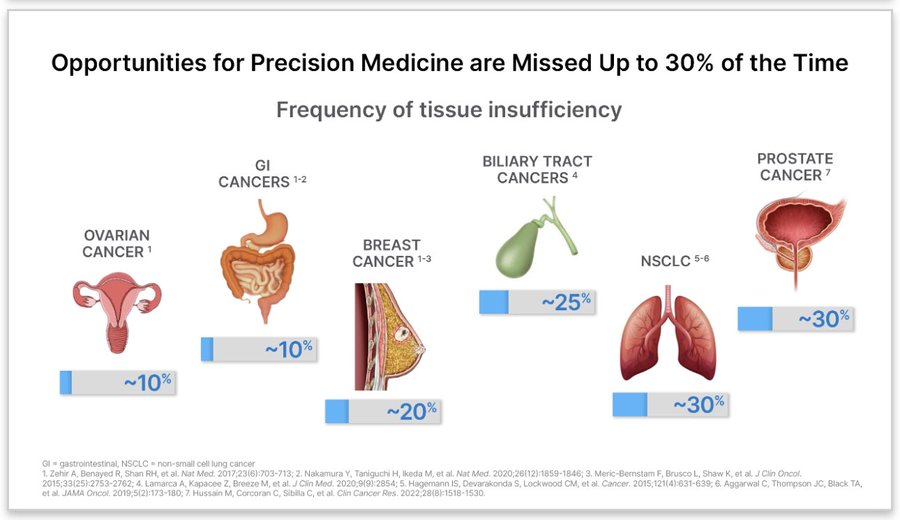
Further tissue testing wasn’t feasible due to low cellularity of the tumor specimen. Opportunities for precision medicine are missed up to a third of the time if not more due to specimen issue, and/or delayed due to tissue not being readily available.
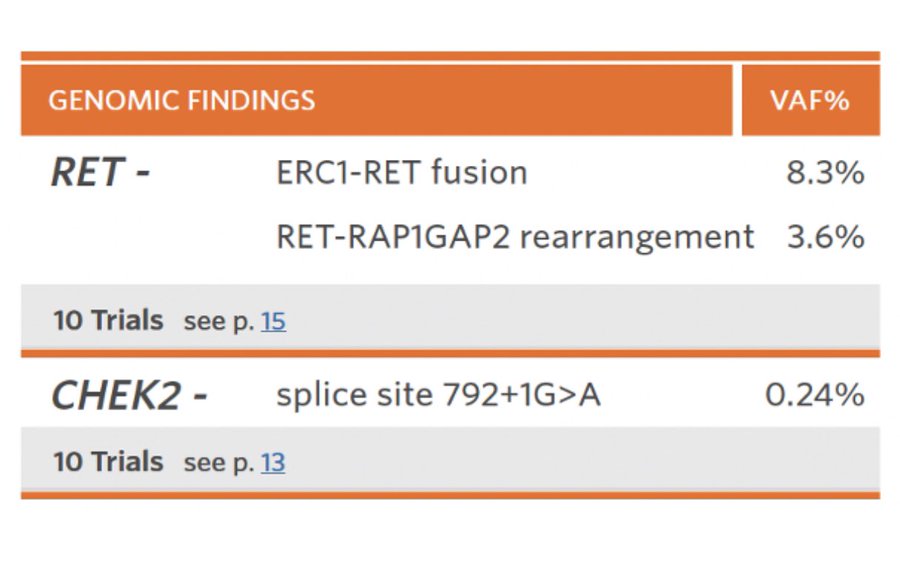
A couple months later I heard back from the family in who were able to send a liquid biopsy here to USA. Guess what we found! A RET Fusion. However, RET fusion inhibitors still not approved in Australia. Precision medicine isn’t complete without access to drugs.
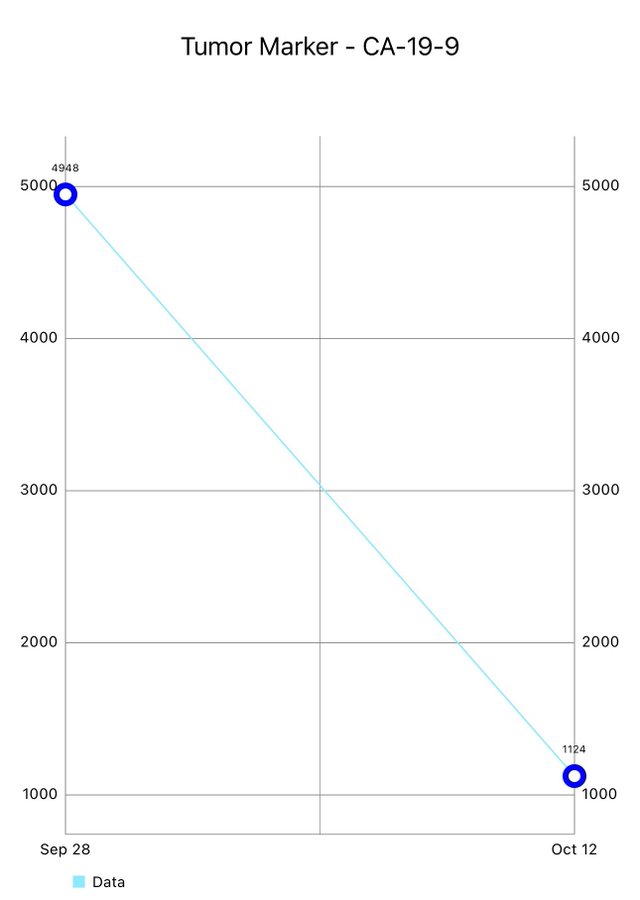
By popular demand, here’s an update: Response to RET after just 2 weeks. From a CA19-9 of nearly 5000 down to ~1000. Not surprising when the driver is a fusion, the responses are brisk, robust, and durable. Remember this is 3rd line.”
Source: Pashtoon Kasi/Twitter
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023