Talha Badar: The story of Luspatercept!
Talha Badar, Assistant Professor of Oncology at Mayo Clinic Comprehensive Cancer Center, recently tweeted:
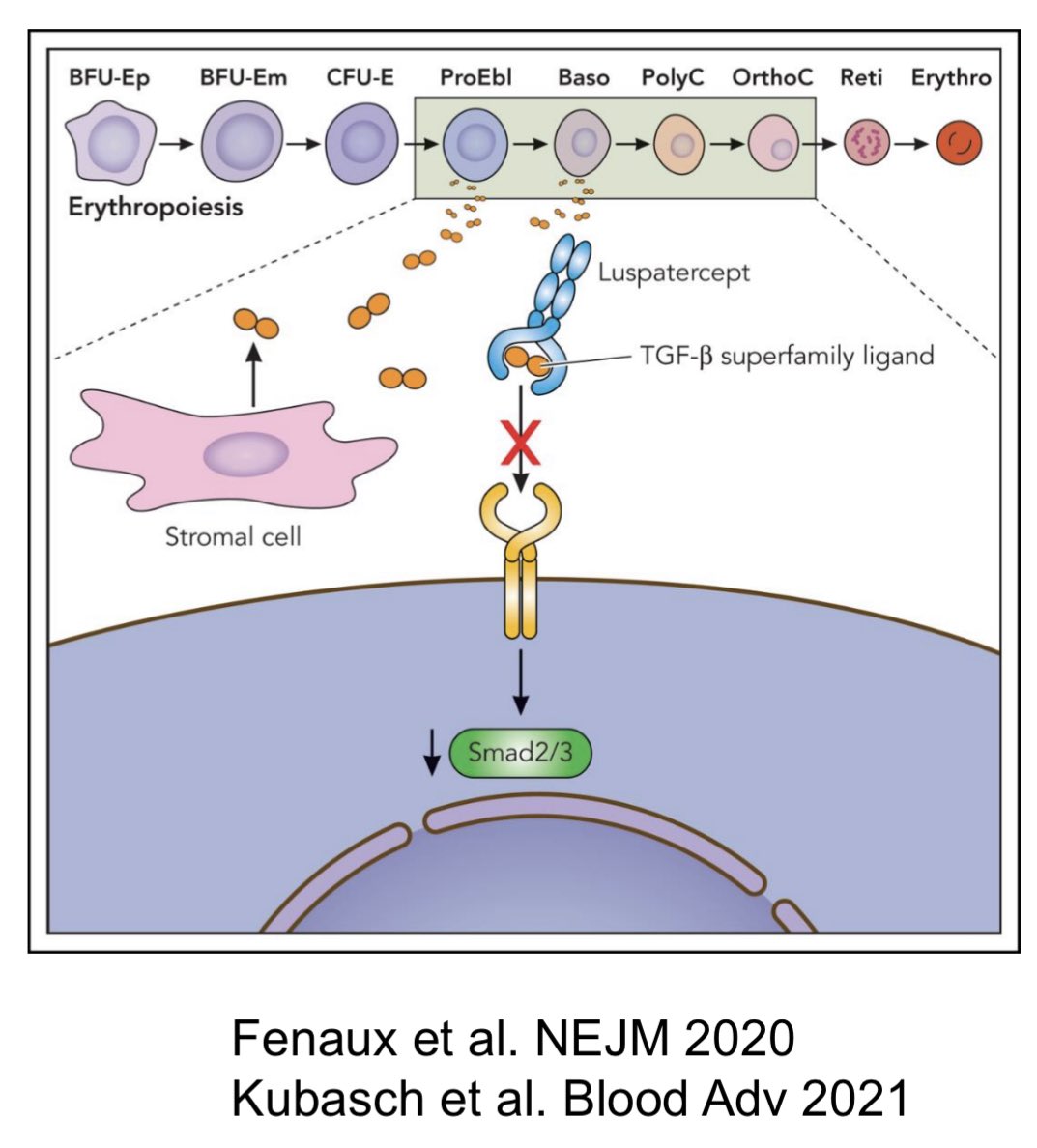
”Weekend review. The story of Luspatercept! Luspatercept, TGF-B fusion trap protein, neutralizes TGF-B and restore effective erythropoiesis.
PACE-MDS trial, PII on 58 LR/IR, TD MDS patients. Luspatercept was given for max 12 wks ( 0.125-1.75 mg/kg). 31/51 pts with higher doses 0.75 to 1.75 had HI-E. MDS-RS, SF3B1m and EPO <200 had favorable responses.
PACE-MDS was followed by P III Medalist trial. MDS-RS ( LR/IR), TD or ref. to ESA, randomized 2:1. 229 patients: luspatercept (153), placebo (76). PE: TI for ≥ 8 wks (b/w wk 1-24). PE was met, got FDA approval.
Followed by COMMANDS trial. Unlike MEDALIST, patients were Rx naive, Not restricted to MDS-RS PE: TI for at least 12 wk, concurrent mean Hb ↑ of at least 1·(wk 1–24) 356 pts randomized: 178 (luspatercept), 178 (ESA) PE was met, got FDA approval.
Real world data suggest better responses in pts with low transfusion burden, HMA/ lenalidomide naive. ”
Source: Talha Badar/Twitter
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
