Luis B Barreiro, Associate Professor of Genetics Section at University of Chicago, shared on X:
“Excited to announce the publication of Sarah Sun’s PhD work in Immunity! Key finding: BCG vaccination reprograms the epigenetic landscape of human bone marrow progenitor cells, reshaping innate immune responses. Full study here in open access.
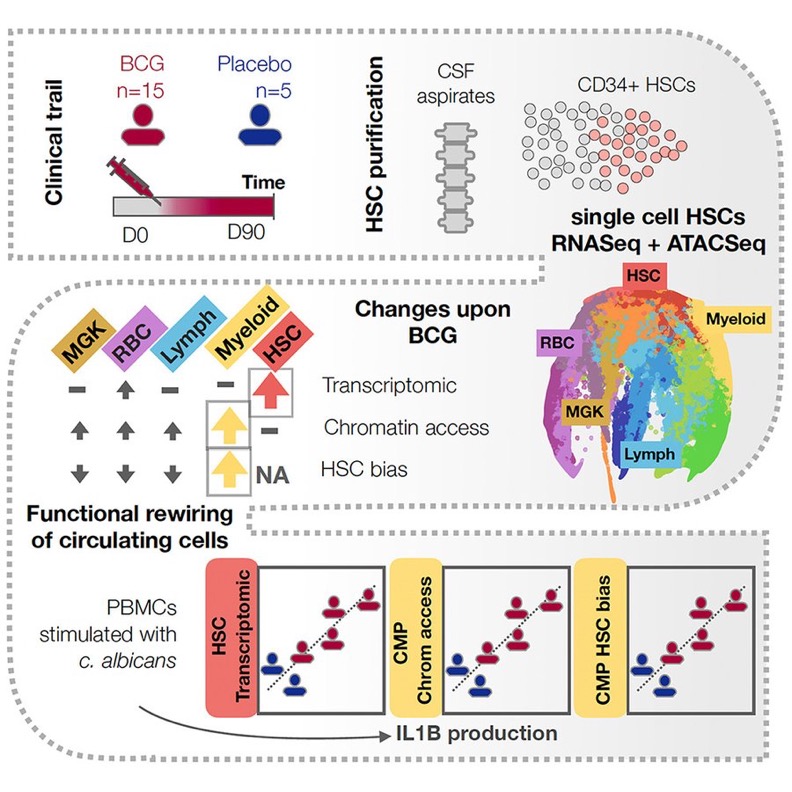
BCG vaccination alters the epigenetic landscape of progenitor cells in human bone marrow to influence innate immune responses
Autors: Sarah J. Sun, Raúl Aguirre-Gamboa, L. Charlotte J. de Bree, Joaquin Sanz, Anne Dumaine, Walter J.F.M. van der Velden, Leo A.B. Joosten, Shabaana Khader, Maziar Divangahi, Mihai G. Netea, Luis B. Barreiro

While BCG is traditionally used to prevent tuberculosis, it also offers protection against a wide range of non-mycobacterial infections. However, the mechanisms behind this protective effect in humans have remained elusive – our work offers valuable new insights into this question.
In our study, we performed single-cell analysis to map gene expression and chromatin dynamics in human bone marrow, before and 90 days after BCG vaccination or placebo. The clinical trial was first described by Cirovic et al. in 2020.
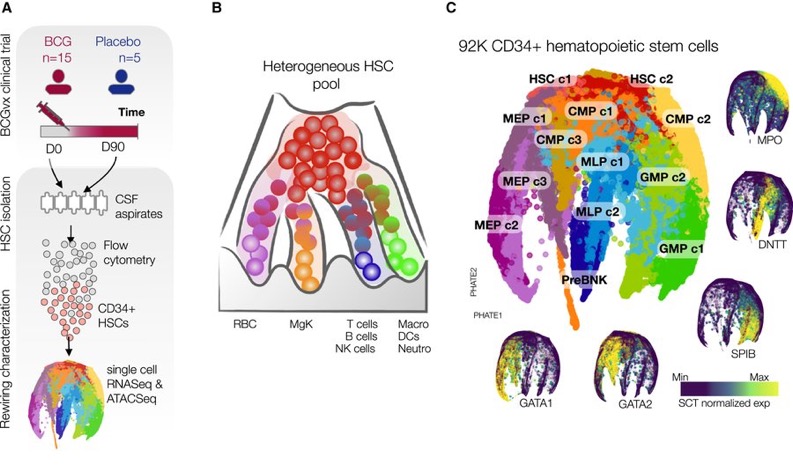
We show that BCG vaccination has a long-term impact on gene expression within hematopoietic stem and progenitor cells (HSPC populations), with the least committed stem cells being the most impacted, followed by megakaryocyte-erythroid progenitors (MEPs).
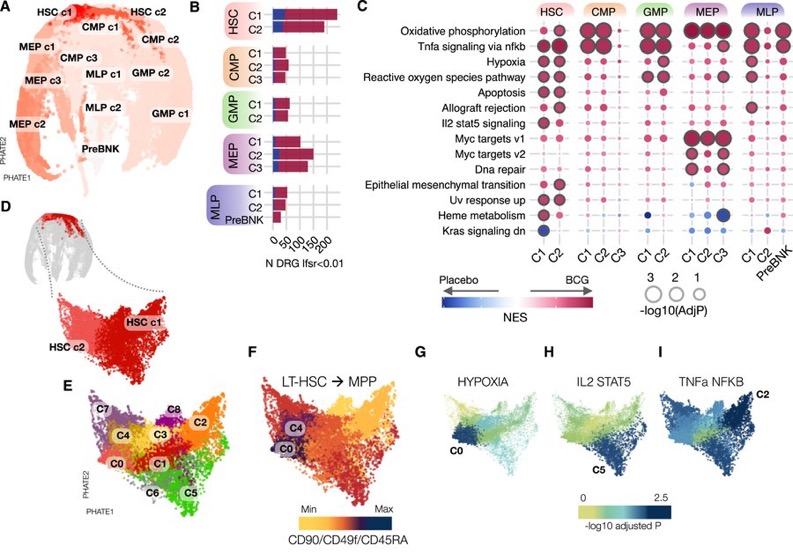
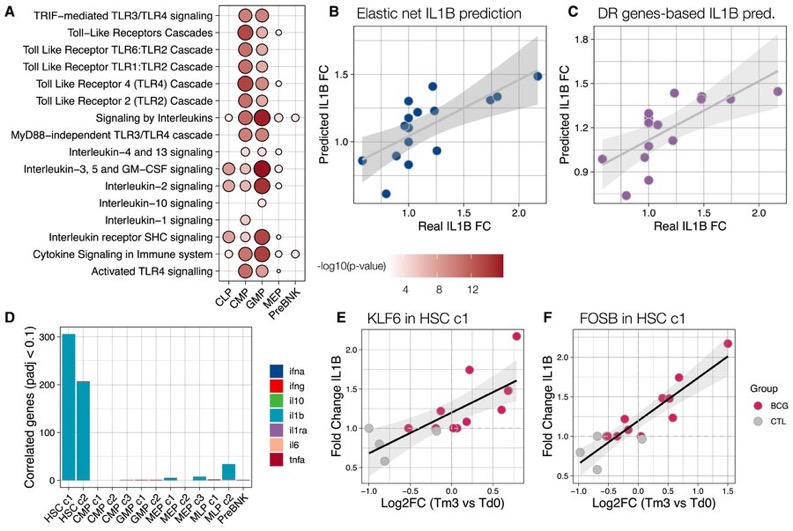
By contrast, chromatin accessibility changes were most prominent in differentiated progenitor cells, particularly at sites regulated by KLF and EGR transcription factors (TFs) —the same TFs driving gene expression changes in stem cells.
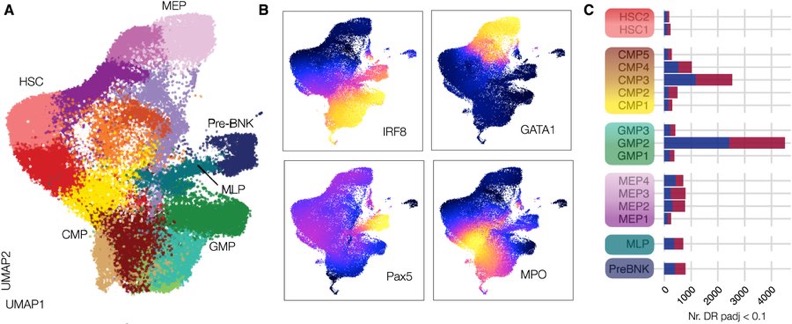
Our model suggests that BCG vaccination tweaks a small set of TFs, leading to a persistent myeloid bias and altered gene expression in HSCs, ultimately reshaping the epigenetic landscape of downstream myeloid clusters.
We then tested if BCG-induced chromatin changes in HSPCs could predict innate immune responses in the periphery. Interestingly, we found that the increase in IL-1β production in response to C. albicans infection could be predicted with high accuracy (R = 0.761, p = 0.001).
Similarly, differential gene expression in HSCs predicted IL-1β responses across donors (R = 0.837, p = 1 × 10−4). Key predictive genes include TFs like KLF6 and FOSB.
Our findings shed light on BCG vaccination’s profound and lasting effects on HSPCs and its influence on innate immune responses and trained immunity.
A complete understanding of the underlying mechanisms will require additional studies in murine models but also advancements in genomics and human challenge models that will allow us to study HSPCs in a much larger number of individuals (e.g., PIE-Seq by Josefowicz Lab).
Congrats to the amazing Sarah Sun and huge thanks to all our amazing collaborators—especially Mihai Netea (not on X) and the team who led the trial, Maziar Divangahi , Shabaana Khader, Raul Aguirre-Gamboa, and Joaquin Sanz. This work wouldn’t have been possible without you all!”
Source: Luis B Barreiro/X
