The European Lung Cancer Congress (ELCC) 2025 held from 26–29 March in Paris, France, brought together over 4,000 participants from across the globe for one of the year’s most impactful gatherings in thoracic oncology. With around 440 abstracts presented, the congress served as a dynamic platform for medical oncologists, radiation oncologists, thoracic surgeons, pneumologists, interventional radiologists, and pathologists to exchange knowledge, debate the latest evidence, and shape the future of lung cancer care.
This year’s meeting continued ELCC’s tradition of fostering cutting-edge science, high-quality education, and international collaboration. From breakthroughs in immunotherapy and targeted treatments to evolving strategies in early detection, precision medicine, and biomarker-driven care, ELCC 2025 delivered a comprehensive overview of progress in the multidisciplinary management of thoracic malignancies.
Among the most discussed presentations were results from the MARIPOSA trial, exploring first-line amivantamab plus lazertinib in EGFR-mutant NSCLC, the AMAZE-Lung trial investigating anti-TIGIT therapy in PD-L1-positive disease, and updated data from KRYSTAL-7, evaluating adagrasib with pembrolizumab in KRASG12C-mutated NSCLC. These landmark studies not only reinforce the growing role of precision medicine in thoracic oncology but also spark new questions on sequencing, combinations, and patient selection in a rapidly evolving treatment landscape.
This year’s Heine H. Hansen Award was presented to Professor Keith Kerr, a distinguished pathologist from the University of Aberdeen, in recognition of his pivotal contributions to lung cancer classification and his role in shaping global guidelines. In his keynote lecture titled “History, Histopathology, and Hyperbole,” Prof. Kerr reflected on the evolving role of pathology in thoracic oncology,
“Pathologists can help translate scientific understanding into clinical practice, bridging the gap between research and patient care.”
His decades-long work – from identifying early immune responses in lung tumors to refining diagnostic classifications – has deeply influenced modern lung cancer management, especially in the era of immunotherapy and precision medicine.’

Our team at OncoDaily has also curated key highlights from ElCC 2025 on social media that you won’t want to miss.
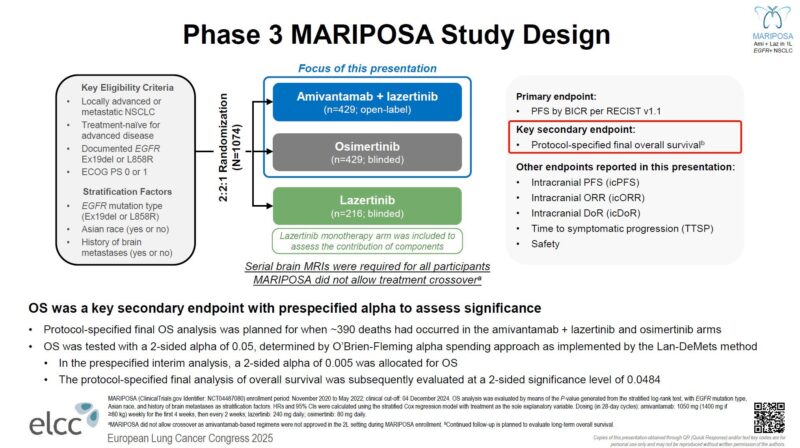
“The MARIPOSA effect.
Awaited OS data for Amivantamab + lazertinib vs osimertinib in 1L EGFRm advanced lung cancer presented by Prof. James Yang at ELCC25.
- OS HR 0.75 (95%CI: 0.61-0.92)
- > 1 year mOS advantage
- No crossover allowed,
- High intracranial activity
- Low toxicity over time w prophylactic mgmt (skin tox)
Is this difference enough to change the standard given extra tox and infusions?”
Alessandra Curioni Fontecedro:
“What a great ELCC2025!
We shared for the first time the findings from the cohort of patients with mesothelioma included in the SAKK17/18 trial:
- 2 years of research to find a synergistic combination for patients resistant to immunotherapy
- 4 Years of conduction and readout of the study
- 8 Centers involved in the study
The study met the primary endpoint:
- In a population of patients where 43% received prior =/>3 lines of therapy
- mPFS 5.6 mt, ORR13% (Indip. radiology review) mOS 9.1 mt
… and broad translational research projects to identify predictive markers.
This is just the beginning: For patients with mesothelioma, we will continue pursuing new approaches.
Thank you to all the patients who participated and their families, to SAKK, Swiss Group for Clinical Cancer Research, and to all colleagues involved in the study.

“At ELCC25, Benjamin Besse from Gustave Roussy delivered an amazing and inspiring presentation on the potential of treatment de-escalation in thoracic oncology.
While drug de-escalation strategies continue to gain traction, challenges—including limited research funding—still hinder their broader implementation. The primary goal of de-escalation is to spare patients unnecessary toxicity and reduce the burden of frequent hospital visits, with the potential added benefit of reducing treatment costs within our health systems.
Immunotherapy, particularly PD-1 and PD-L1 inhibitors, has been a major focus for de-escalation due to their pharmacokinetics, which allows flexibility in dosing. Three main approaches have been explored:
- Reducing treatment duration: Although PD-1/PD-L1 inhibitors are often prescribed for at least two years, retrospective studies suggest no clear benefit to continuing beyond this period. The DIAL trial (NCT05255302) is currently investigating whether stopping treatment after six months, with the option to reintroduce it upon progression, might yield similar outcomes.
- Extending dosing intervals: Trials such as the French PULSE study and the UK’s REFINE-Lung trial (NCT05085028) are evaluating whether immunotherapy can be given less frequently while maintaining efficacy.
- Lowering the dose: Some early studies suggest that much lower doses of certain immunotherapies may still provide clinical benefit, particularly in resource-constrained settings. However, cost savings depend on drug pricing models, which do not always align with dose reductions.
While these strategies hold promise, de-escalation must be grounded in strong biological rationale and tailored to each drug class.
So, what’s needed to move the field forward?
- Biomarker research to identify which patients can safely undergo de-escalation.
- Greater investment in de-escalation trials, as long-term cost savings could offset trial expenses.
- Regulatory adaptation to ensure evidence-based dosing changes are incorporated into guidelines.
Collaboration among researchers, clinicians, industry, and regulators will be key to making drug de-escalation a safe and effective reality.”
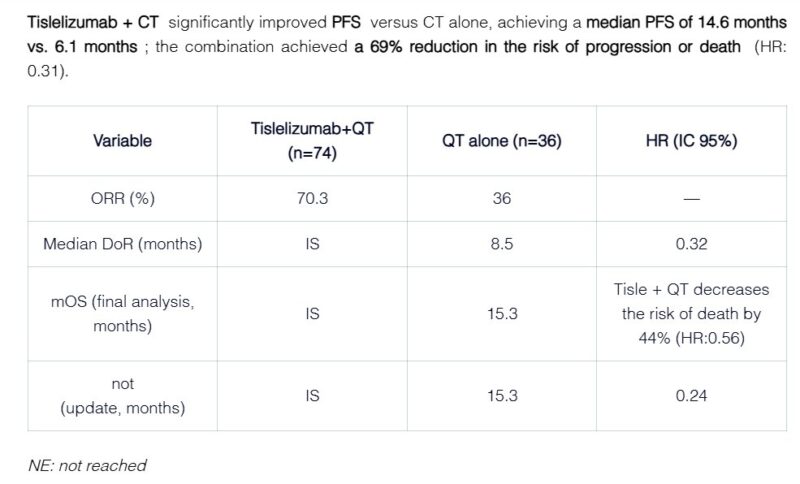
“Tislelizumab + QT: A New Hope in NSCLC.
At ELCC2025, Dr. Yan Yu presented an analysis from the Phase III RATIONALE-304 study, focused on this subpopulation with high potential for immunotherapeutic response.
Objective: Evaluate the efficacy of tislelizumab combined with chemotherapy (QT) in patients with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC).
Inclusion Criteria:
- Diagnosis of advanced or metastatic non-squamous NSCLC
- PD-L1 expression ≥50% in tumor cells
- No EGFR or ALK mutations
Key Findings: Progression-Free Survival (PFS): Median PFS of 14.6 months with tislelizumab + QT vs. 4.6 months with QT alone.
Objective Response Rate (ORR): Higher response rates with the combination of tislelizumab and QT.
Safety: Most common grade ≥3 adverse events: neutropenia and leukopenia, primarily associated with QT.
Conclusion: The combination of tislelizumab and chemotherapy significantly improves PFS and response rates in PD-L1 high-expression NSCLC, offering a promising therapeutic option.”
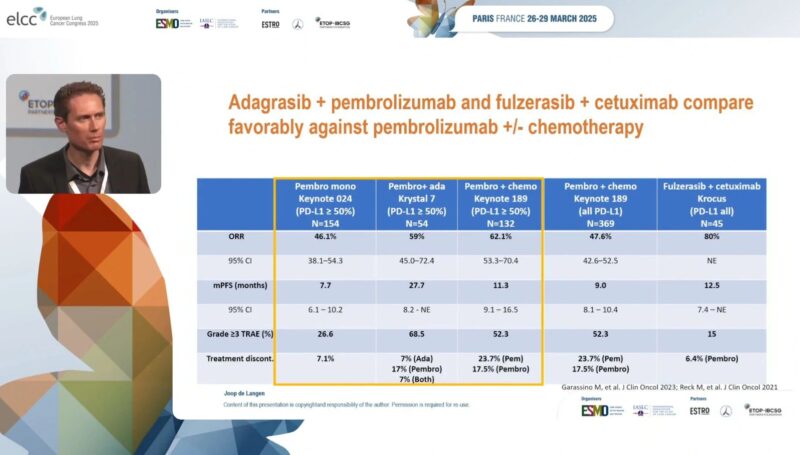
“Can we improve outcomes of KRAS mutated advanced NSCLC? What’s the new data?
Check the slide to compare the results of adagrasib plus pembrolizumab and Fulzerasib plus Cetuximab with the current SOC. Money slide!
Daraxonrasib. New first-in-class pan RAS inhibitor. Compared with Adagrasib and Sotorasib in NSCLC.”
ESMO:
“Clinical trials presented at ELCC25 evaluated the efficacy and safety of new treatments for advanced/metastatic RAS-mutated NSCLC.
According to updated data from the KRYSTAL-7 phase II trial, adagrasib plus pembrolizumab continued to have encouraging efficacy in patients with KRASG12C-mutated NSCLC and PD-L1 ≥50%. Median progression-free survival (PFS) was 27.7 months but high rates of grade ≥3 adverse events were noted.
In an updated analysis from the KROCUS phase II trial investigating fulzerasib plus cetuximab in untreated patients with KRASG12C-mutated NSCLC, the median PFS was 12.5 months with consistent results across levels of PD-L1 expression and manageable toxicity.
Promising phase I/Ib trial findings were also presented for daraxonrasib in previously treated RAS-mutated NSCLC.
Read the full news article here.”
“ELCC25 Mini Orals.
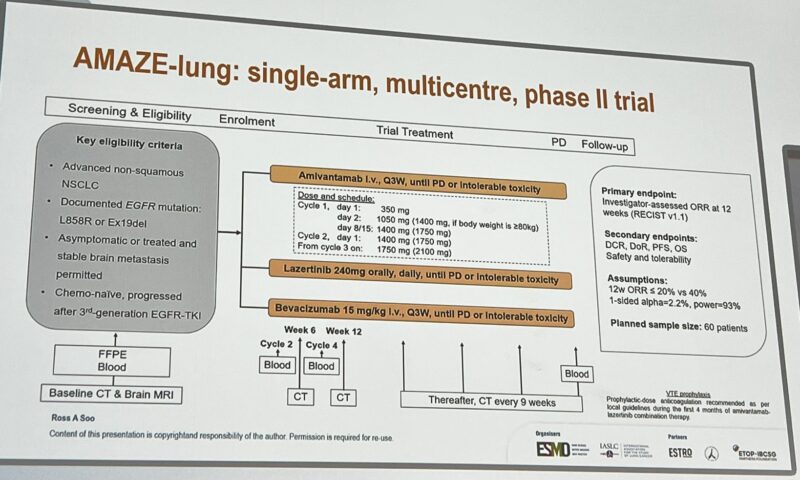
Phase II AMAZE-Lung Trial: 2L ami-laz-bev post 3rd generation TKI by Ross Soo.
- 61 patients
- ORR at 12 weeks – 33%
- mDOR 13.9m, mPFS 10.9m, mOS 15.5m
An example of targeting VEGF in EGFR+ NSCLC is another chemo-free approach. ”
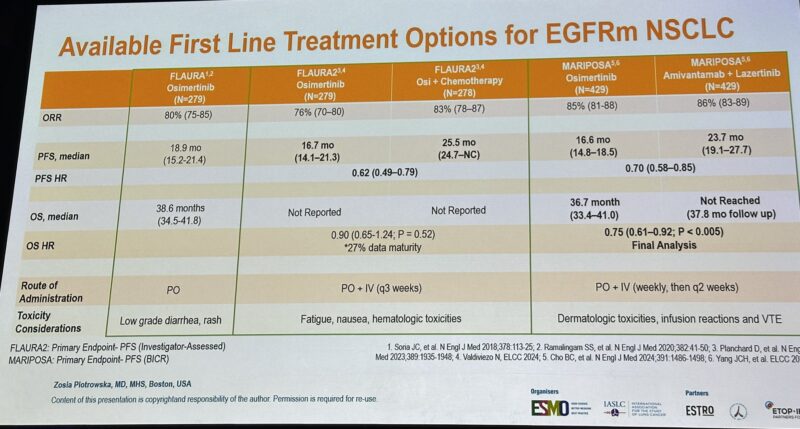
“An excellent debate here at ELCC25 on the optimal choice for 1st Line treatment of EGFR pos NSCLC.
Osimertinib monotherapy vs Combinations (FLAURA2/MARIPOSA).
Insightful lectures by Zosia Piotrowska and Antonio Passaro, focusing on efficacy endpoints, biology, toxicity, and cost-effectiveness. The winner is always the consideration of genetic and clinical risk factors, individualized discussion, and personalized approach.”
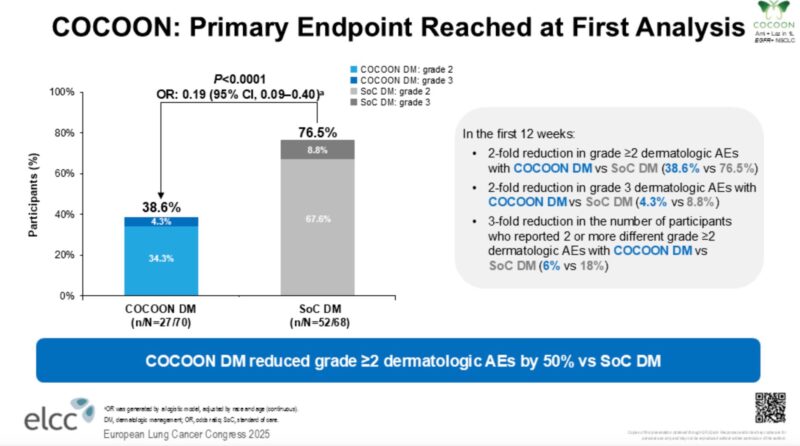
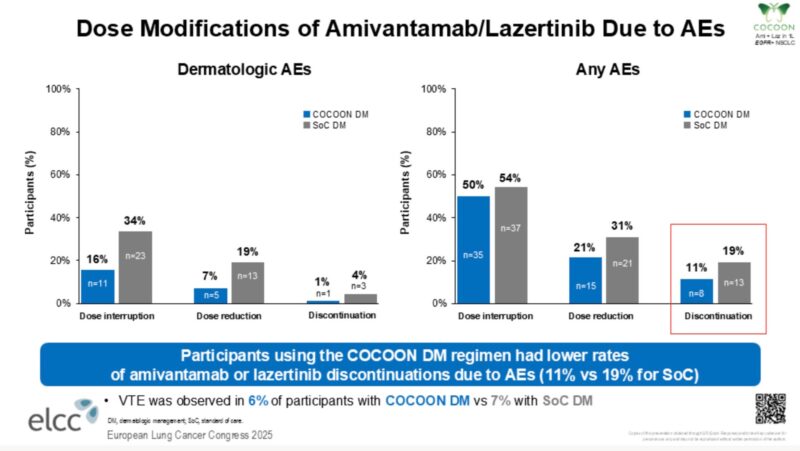
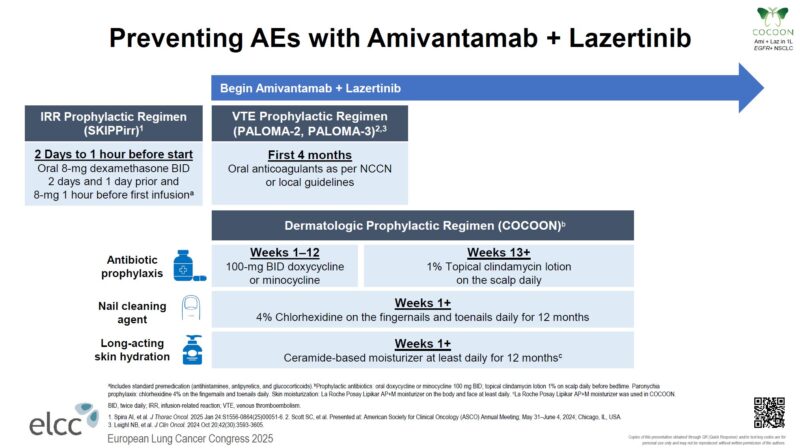
“COCOON data at ELCC25 — can prophylactic enhanced dermatologic management prevent moderate to severe dermatological AEs with amivantamab + lazertinib?
COCOON reaches the primary endpoint.
Within the first 12 weeks,
- G2+ dermatologic AEs decreased from 76.5% to 38.6%.
- G3 dermatologic AEs decreased from 8.8% to 4.3%.
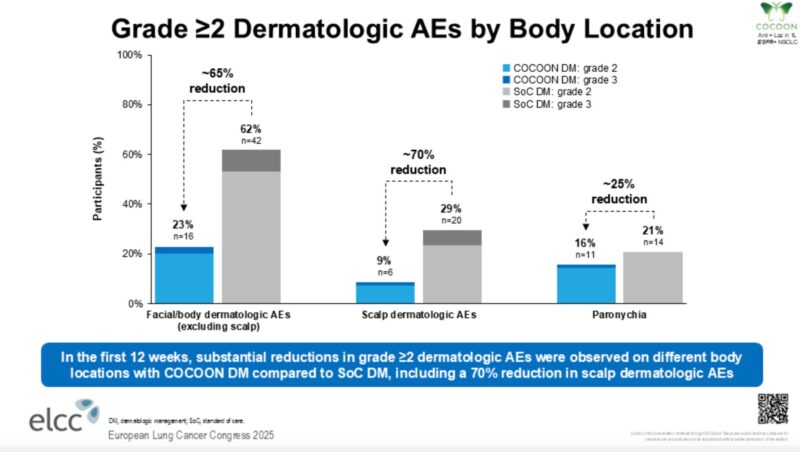
Meaningful slide—scalp lesions remain one of the most challenging dermatologic AEs with this combination.
Encouraging to see a ~70% reduction in G2+ scalp dermatologic AEs (29% v 9%), though small numbers.
Encouraging to see that patients who received prophylactic-enhanced dermatologic management had lower AE-related discontinuation rates (11% v 19%).
COCOON and MARIPOSA OS data, my thoughts:
- The hand that writes MARIPOSA must write a dermatology consult and the prophylactic COCOON regimen.
- Supportive care can effectively keep patients on treatment and is essential to fully realize the benefit we aim for. “
“I have many thoughts (surprise), but ultimately, there isn’t a one-size-fits-all treatment. It’s unsettling that opinions are being formed already based on side effects without seeing the COCOON data that will be presented today.
I am a passionate and vocal advocate for QOL, but we can’t ignore the survival data. This isn’t about gaining a few extra months’ – 12+ months is significant and should not be overlooked!
I can’t help but think personally about:
1. My dad would have lived to see me graduate 8th grade (he died a few weeks before), maybe even high school.
2. My mom would have lived to see the birth of my daughter (she died 6 months before) & likely all of my children.
That MATTERS, too!
While I can’t say what my parents would have chosen, it is ultimately the patient’s/family’s choice! Every family deserves the information and opportunity to discuss ALL options (Informed decision-making). This respect for patient choice should be a priority.
I also feel strongly that the COCOON data should have been presented alongside the MARIPOSA data. It’s just as crucial (even more) because it addresses the significant barrier of side effects that impact whether a patient can stay on treatment. Kudos to J&J for listening to what’s important to patients and developing a study to address the side effects up front!
If QOL truly matters, then so should the data that addresses it! Presenting them together tells a much bigger story and would have changed the conversation. I implore researchers, clinicians, and conference organizers to think about this going forward.”
ESMO:
“As presented at ELCC25, subcutaneous (SC) pembrolizumab – consisting of pembrolizumab co-formulated with berahyaluronidase alfa – demonstrated noninferiority in pharmacokinetics compared with intravenous (IV) pembrolizumab in the phase III MK-3475A-D77 trial. Similar efficacy outcomes were observed.
Patients may prefer SC immunotherapy administration and other potential benefits include reductions in pharmacy preparation, nursing administration time, infusion-chair occupancy, and infusion-related adverse events than with IV administration.
Results plus commentary in the ESMO Daily Reporter.”

“NOW OUT at ELCC25!
COCOON: Preventing Moderate to Severe Dermatologic Adverse Events in First-line EGFR-mutant Advanced NSCLC Treated with Amivantamab Plus Lazertinib.
- AEs ≥G2: OR 0.19 (95% CI, 0.09–0.40)
- NCT06120140.”
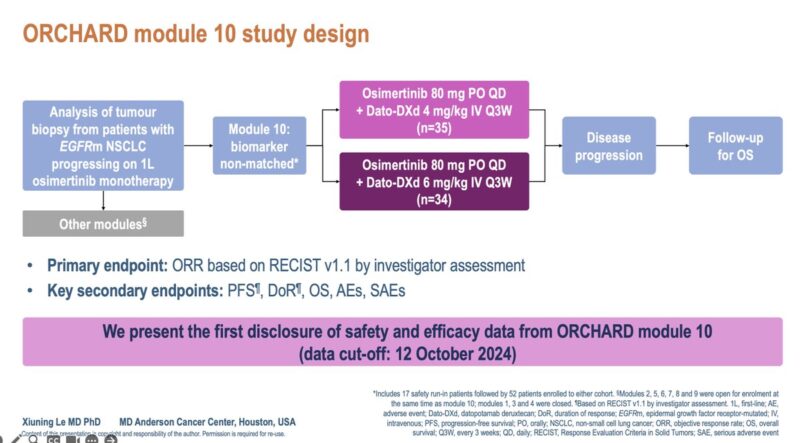
“A very successful ELCC25.
I presented Dato-DXd (4mg/kg vs 6mg/kg) with osimertinib in EGFR refractory NSCLC patients, from the ORCHARD trial.
mPFS is 11.7 months and ORR 36% in the Dato-DXd 6mg/kg cohort. Improving upon what we have and exploring new combinations.”
“Honored to moderate the session ‘Above RECIST: A new role for MRD and cfDNA in tumor assessment’ alongside Professor Thierry Berghmans at ESMO – European Society for Medical Oncology ‘European Lung Cancer Congress’ – ELCC2025 held in Paris, France, from March 26-29 2025.
I opened the session with a lecture on ‘Liquid biopsy: Principles and pitfalls for the clinician’ in the Plenary Room. I highlighted our work on Liquid Biopsy and Lung Cancer at the Recherche et Innovation – CHU de Montpellier. Thousands of participants were present!
This was followed by outstanding presentations by Thomas John (Melbourne, Australia), Atocha Romero (Majadahonda, Spain), and Heather Wakelee (Stanford, USA).
A truly enriching experience with great interest and enthusiasm around this emerging topic!
I met many friends and colleagues!”

“Heterogeneity of resistance mechanisms should lead to a personalized strategy to overcome resistance. Great and subtle presentation by Laura Mezquita at ELCC2025.
‘Smart thinking’ is bigger than the same treatment/sequence for all.”
The ELCC 2026 will take place from March 25 to 28 in Copenhagen, Denmark. Stay tuned to OncoDaily for more updates.


