The 2025 ASCO Gastrointestinal (GI) Cancers Symposium took place from Thursday, January 23 to Saturday, January 25.
This event featured the latest research, treatments, and strategies in the fight against GI cancers. People had the chance to attend expert-led sessions on emerging therapies, clinical breakthroughs, and multidisciplinary approaches to patient care.
For more details on the program, check out the official Meeting Announcement and download the full schedule.

Our team at OncoDaily has selected a few highlights from ASCO GI 2025 that you should not miss:
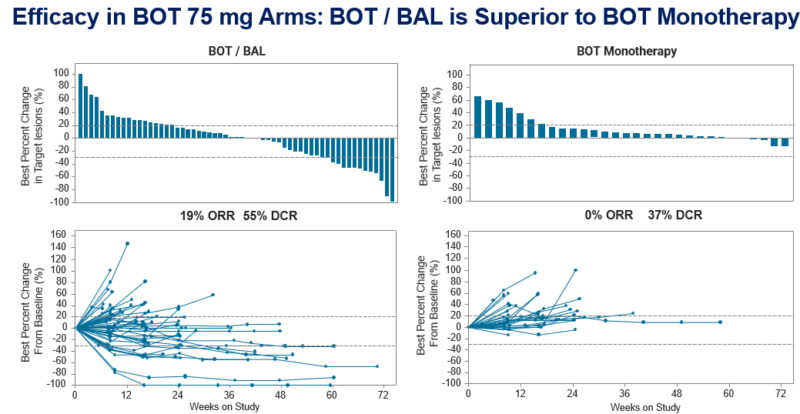
“Today, at GI ASCO, I had the privilege of presenting the results of R Phase 2 study of BOT +/- BAL in chemoresistant MSS CRC- an area in dire need of treatment options. BOT75/BAL240 ORR 19%, DCR 55%. BAL value confirmed. Majority study responses ongoing. DURABLE responses matter.”
“Started my day as a Session co-chair ASCO GI Cancers 2025 Symposium with Alexander Parikh at San Francisco.
‘Practical Considerations for the Management of Pancreatic and Biliary Tumors.’
- Patient performance status.
- Geriatric assessment.
- No treatment is treatment.”

“GI25 Micdrop NEST:
NO RECURRENCES! Neoadjuvant immunotherapy keeps delivering! Patients with MSS, NOT just MSI-High in colorectal cancer. Goal is to CURE more patients. Phase2/3 soon.
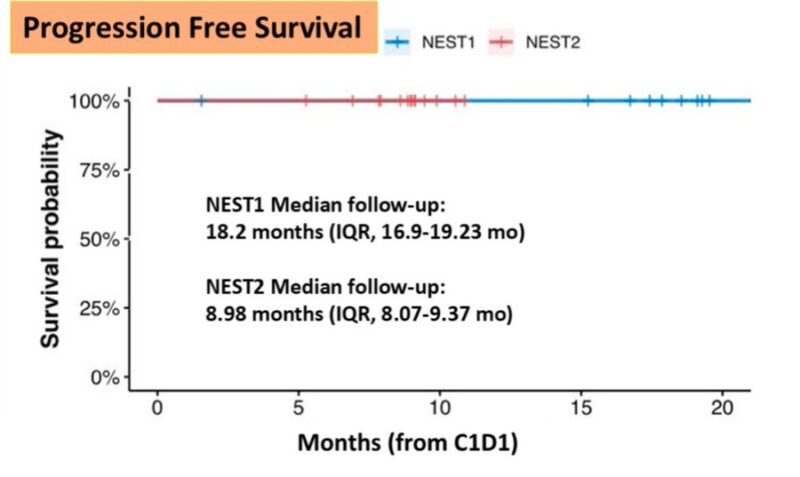
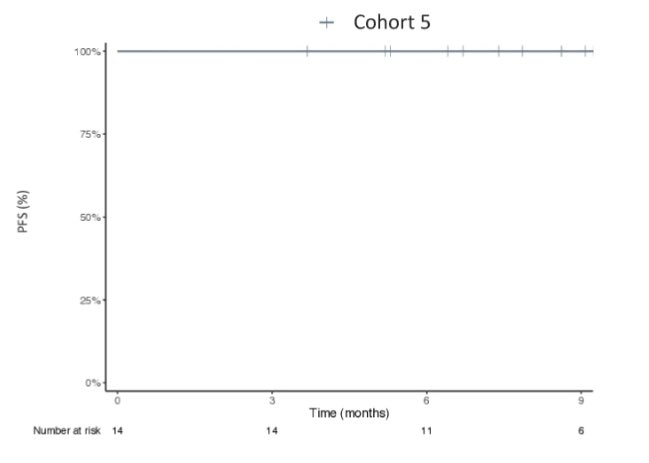
The UNICORN study of BOT +/-BAL corroborates the findings of our NEST trial. Also provides: “Contribution of Components”. BOT needs its partner BAL. Not entirely surprising since earlier data on IPI/NIVO also showed that CTLA4 was not sufficient.
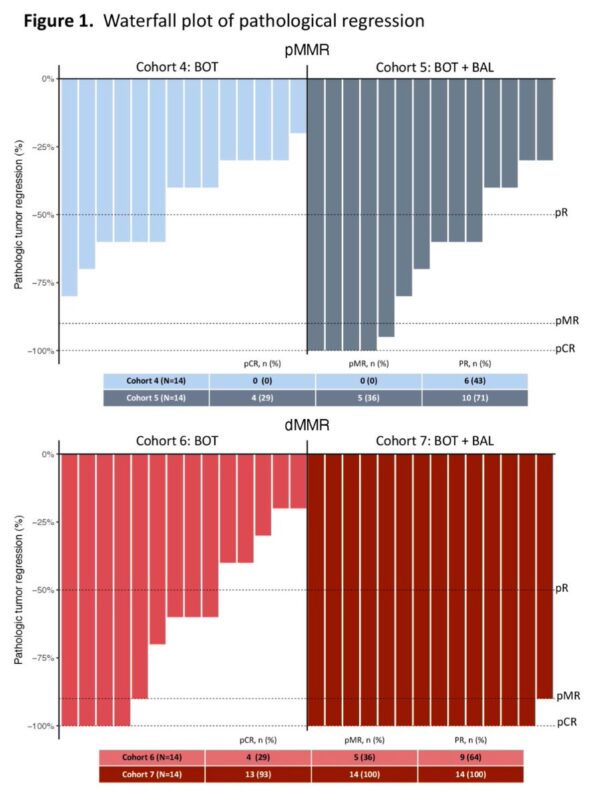
Here’s the replica of the flatline from the UNICORN study, as noted in NEST trial as well. From BOT BAL arm for patients with MSS colorectal cancer Again, reiterating the larger goal and unmet need of CURING more patients.”
“Letter to my congressman:
This week, I have been attending GI25, a scientific conference focused on research advancements in GI cancers (pancreatic, colorectal, gastric, biliary tract, and others). I am here to support the work of the researchers whose clinical trials my company manages, and to help generate new opportunities for collaboration.
In the midst of this conference, during a break between meetings, I read the headlines that the new administration had cancelled all NCI and NIH section meetings, communications, scientific publication, hirings, and other activities.
There are thousands of cancer researchers who collaborate with their federal peers, and whose work relies on the resources provided by these agencies. All of their vital activity under the NIH umbrella came to an immediate halt in real time during this conference.
This is not hyperbole, and while I wish this were simply the plot for a fictional thriller, it is our present reality. Every day this persists is a day of progress lost, and consequently a longer path toward badly needed advancements in research.
Our friends, neighbors, and family members who battle chronic or deadly disease deserve better.”
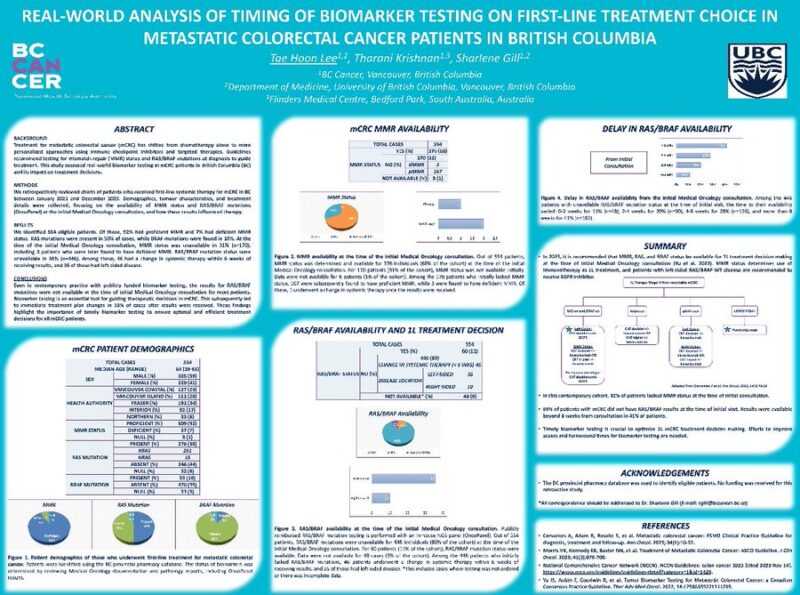
“Access to timely biomarker testing remains a challenge for 1L, mCRC.
Our review confirmed RAS-BRAF negative available at MO consult 80% of the time.
Strategies to improve timely biomarker testing are needed.
Congrats to our BC Cancer resident Tae Hoon Lee.”
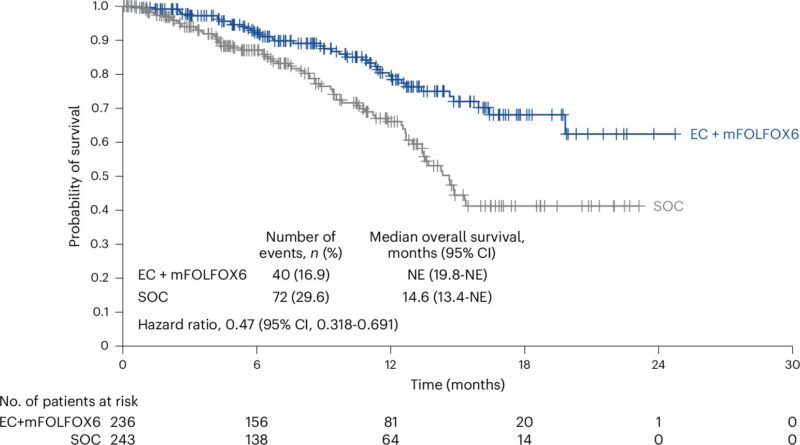
“Exciting data from BESPOKE CRC indicates that ctDNA based surveillance can lead to an increased role for metastasis-directed therapy in colorectal cancer.”

“Straight from GI25 in Nature Medicine.
By Scott Kopetz, Cathy Eng and colleagues.
Encorafenib, cetuximab and chemotherapy in BRAF-mutant colorectal cancer: a randomized phase 3 trial.
Trial met one of the dual primary endpoints (objective response rate) with PFS endpoint still ongoing. The BRAF mutated subset of CRC is a tough, tough molecular subset. Advances are welcome.”
“Thing to look at ASCO each year is the ‘incremental’ gains in survival for our patients with MSS colorectal cancer.
A few years ago I started seeing survivals >40+ months. This year I’m seeing >50-52 months.
For MSI, ‘not reached’ is a recurring theme.”
“Sharing during GI25 with extraordinary clinical scientists including Pamela Kunz and Crystal Delinger CEO, National Comprehensive Cancer Network (NCCN).
Our PanOncology investigators including Noridza Rivera presented 5 scientific abstracts.
long term follow up with KRAS+, colon cancer.
ctDNA analysis for surveillance.
MET-inhibitors for Stage IV colon cancer.
anti-PD1 therapies for 1st line therapy for gastric cancer.”

“As GI-ASCO comes to a close I think there are a few takeaways.
1. Progress continues to march forward. dMMR disease is increasingly a systemically managed disease with excellent cure fraction (embrace CTLA4).
2. IO is breaking into MSS CRC.
3. The undruggable is becoming druggable.
4. Aspirin and Celecoxib may have clinical benefit!?
5. ctDNA is here to stay, but we need to identify actionable strategies.”
“Congrats Daniel Haldar for your ASCO GI25 master class on where we are and where we are heading for cancer vaccines in MSS CRC – excited to see where you lead the field to end cancer for our crcsm patients in the years to come!”
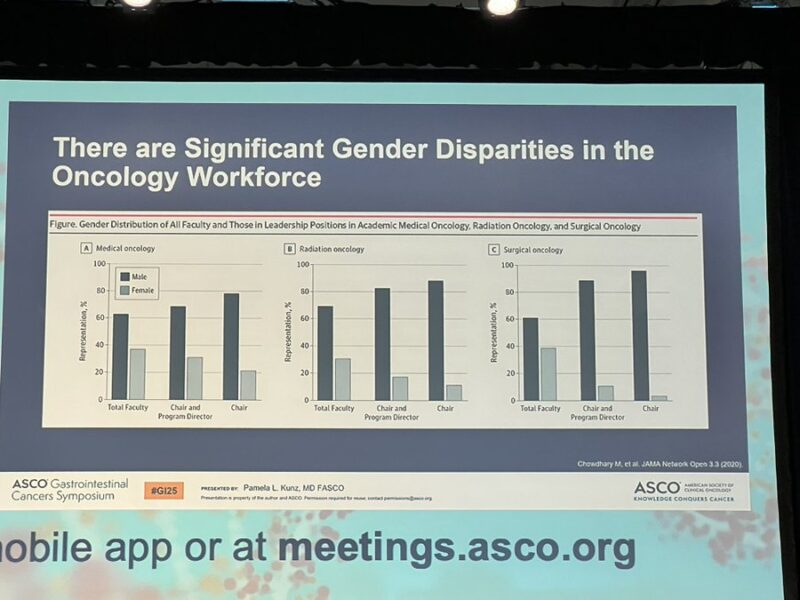
“So disheartening to see the data of gender disparities in oncology leadership. I also recognize the enormous privilege I have had thus far due to amazing he for she mentors/sponsors such as Jordan Berlin and Tanios Bekaii-Saab among others. I know not everyone does.”
“Opening keynote lecture, Pamela Kunz.
‘Disrupting GI Oncology: Shattering Barriers with Inclusive Science’.
Highlighting documentary film.”

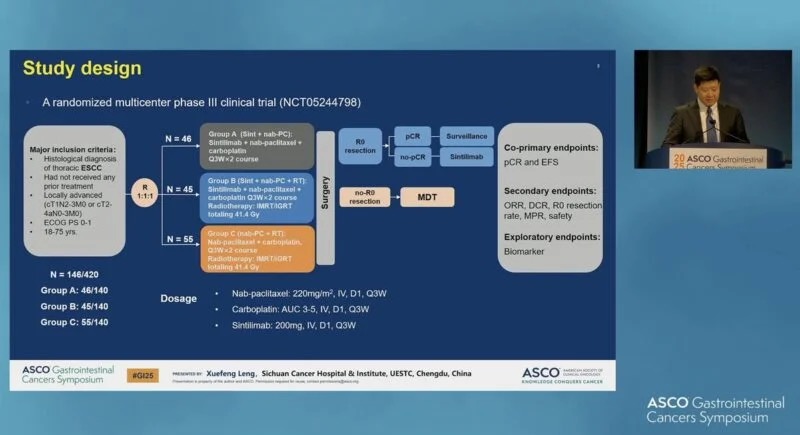
“Xuefeng Leng presents neoadjuvant chemoRT +/- sintilimab for ESCC.
Statistically significant improvement in pCR rates…but is this similar to the reduction in disease we see with CHECKMATE-577?
Will need long term survival data for both studies to see if sequence matters.”
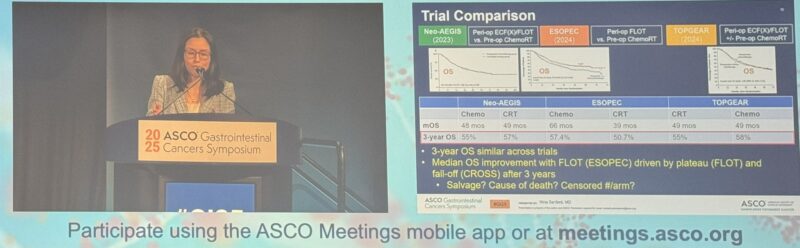
“Nina Niu Sanford dropping pearls of wisdom synthesizing the literature to guide contemporary management of esophagus cancer.
Takeaways here!
- peri-op chemo vs CRT trial comparisons.
- contemporary treatment algorithm.
- role of organ preservation to improve QoL.
Brilliant talk!”
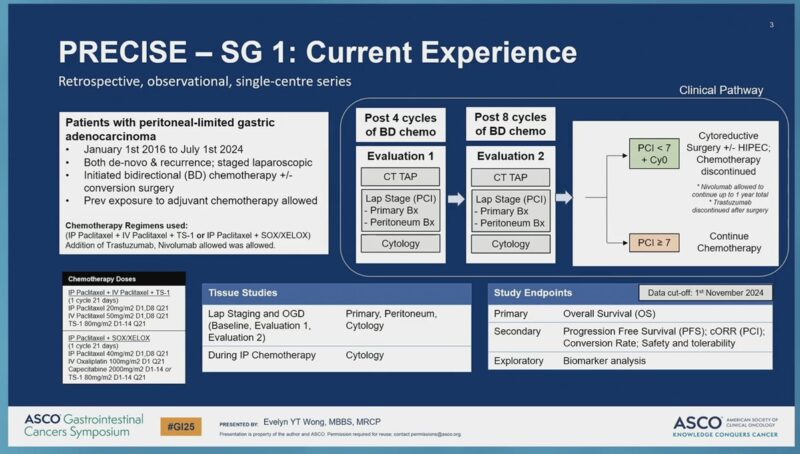
“Outcomes of patients with peritoneal-limited metastatic gastric cancer undergoing bidirectional CTx cytoreductive surgery with HIPEC, ASCOGI25.
- PRECISE-SG 1, single center RWD.
- mPFS 9.5.
- mOS 17.5mo.
- Surgery associated with better OS.”
“CARES-005 is positive! Another IO-TACE positive trial…but doubts remain.
PFS with TACE: 3.2 months only, OS negative, phase 2.”

“Another trial unfortunately negative for our patients with pancreas cancer.
As mentioned, innovation is needed.
The PanCAN is a great platform to study novel drugs.
While mechanistically, Pamrevlumab was of value to study, it didn’t improve survival.”
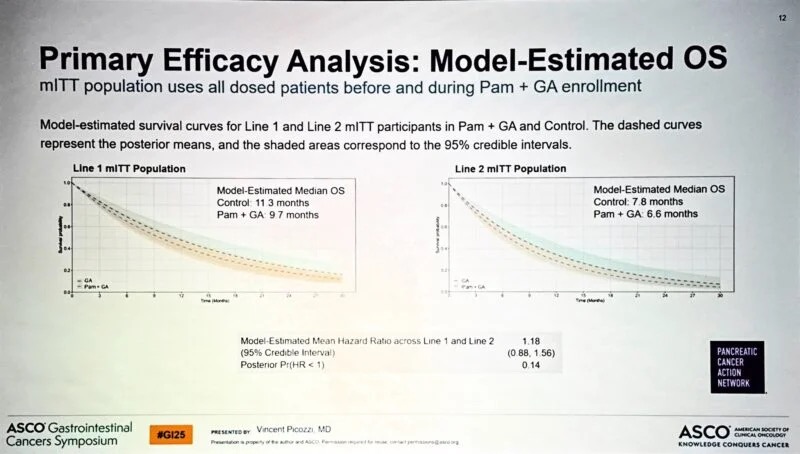
“Lipika Goyal, Stanford Cancer Institute gives an excellent overview of both the history and principles of adjuvant therapy (in the context of HCC).
Asking patients to accept non-zero toxicity for unknown benefit ought to be one of the most scrutinized value propositions in oncology.”
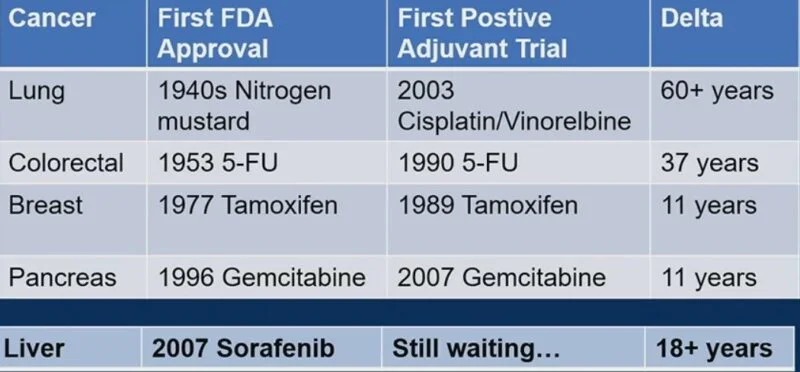
“Nice HCC debate and discussion by Lauren Henke.
Historically (neo)adjuvant trials in HCC have been designed around resection and ablation.
With high rates of control/ablative doses achievable with rad seg and SBRT, argue these modalities should also be included as definitive options.”

Highlights from ASCOGI25 Day 1


