The 2025 ASCO Gastrointestinal (GI) Cancers Symposium took place from Thursday, January 23 to Saturday, January 25.

This event featured the latest research, treatments, and strategies in the fight against GI cancers. People had the chance to attend expert-led sessions on emerging therapies, clinical breakthroughs, and multidisciplinary approaches to patient care.
For more details on the program, check out the official Meeting Announcement and download the full schedule.
Our team at OncoDaily has selected a few highlights from ASCO GI 2025 Day 3 that you should not miss:
“ctDNA in CRC is prognostic, but there is no data showing that treatment initiated earlier based upon ctDNA positivity confers improved survival (or QOL).
Earlier detection alone does not equal benefit.
Great job Kristen Ciombor.”

“Thing to look at ASCO each year is the ‘incremental’ gains in survival for our patients with MSS colorectal cancer.
A few years ago I started seeing survivals >40+ months. This year I’m seeing >50-52 months.
For MSI, ‘not reached’ is a recurring theme.”
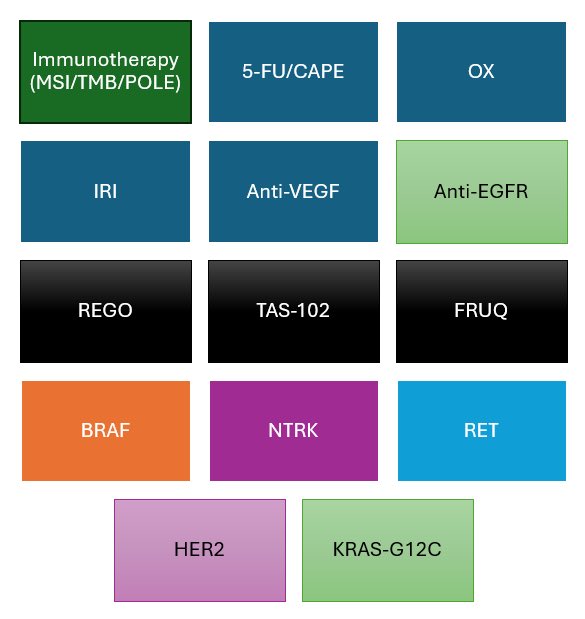
“Prognostic significance of detectable ctDNA in the NEO-trial.
NEO evaluated 3m FOLFOX local excision for T1-3bN0 rectal cancer
Study suggests detectable ctDNA (guardant reveal) was associated with poorer response and persistent cancer.”
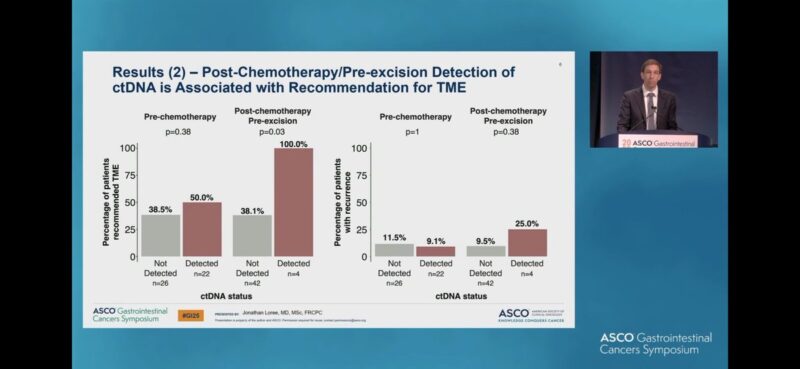
“Access to timely biomarker testing remains a challenge for 1L mCRC.
Our review confirmed RAS-BRAF available at MO consult 80% of the time.
Strategies to improve timely biomarker testing are needed.
Congrats to our BC Cancer resident Tae Hoon Lee.”
“Great discussion on updates in anal cancer by Cathy Eng.
Anal cancer incidence rising, early stage most common. 5-year OS 36%
Locally advanced anal cancer:
-No role for radiosensitizing chemo- RTOG98-11, ACTII
-5-FU-MMC remains SOC (cisplatin may be more appropriate in immunosupp pts, 5-FU in frail pts)
-no randomized data, but cape can be considered as an alternative to 5-FU, myelosupp may be an issue.
-IO in this setting-no role, ongoing studies.
-no role for adjuvant or maintenance therapy.
Advanced anal cancer:
-InterAACT: carbo-taxol became SOC due to similar ORR and better safety profile.
-POD1UM-303: Retifanlimab+carbo+taxol (compared to carbo+taxol) in metastatic treatment naive metastatic anal SCC: PFS improvement, ORR 56% trend for OS improvement. HIV + pts included. Now an NCCN 2B recommendation.
-EA2176: Carbo/taxol with or without Nivo- PFS primary endpoint, ctDNA incorporated. Finished enrolment. HIV+ patients included.”

“Straight from GI25 in Nature Medicine.
By Scott Kopetz, Cathy Eng and colleagues
Encorafenib, cetuximab and chemotherapy in BRAF-mutant colorectal cancer: a randomized phase 3 trial.
Trial met one of the dual primary endpoints (objective response rate) with PFS endpoint still ongoing. The BRAF mutated subset of CRC is a tough, tough molecular subset. Advances are welcome.”
“Letter to my congressman:
This week, I have been attending GI25, a scientific conference, The American Society of Clinical Oncology’s annual meeting focused on research advancements in GI cancers (pancreatic, colorectal, gastric, biliary tract and others).
I am here to support the work of the researchers whose clinical trials my company manages, and to help generate new opportunities for collaboration.
In the midst of this conference, during a break between meetings, I read the headlines that the new administration had cancelled all NCI and NIH section meetings, communications, scientific publication, hirings, and other activities.
There are thousands of cancer researchers who collaborate with their federal peers, and whose work relies on the resources provided by these agencies. All of their vital activity under the NIH umbrella came to an immediate halt in real time during this conference.
This is not hyperbole, and while I wish this were simply the plot for a fictional thriller, it is our present reality. Every day this persists is a day of progress lost, and consequently a longer path toward badly needed advancements in research.
Our friends, neighbors, and family members who battle chronic or deadly disease deserve better.”
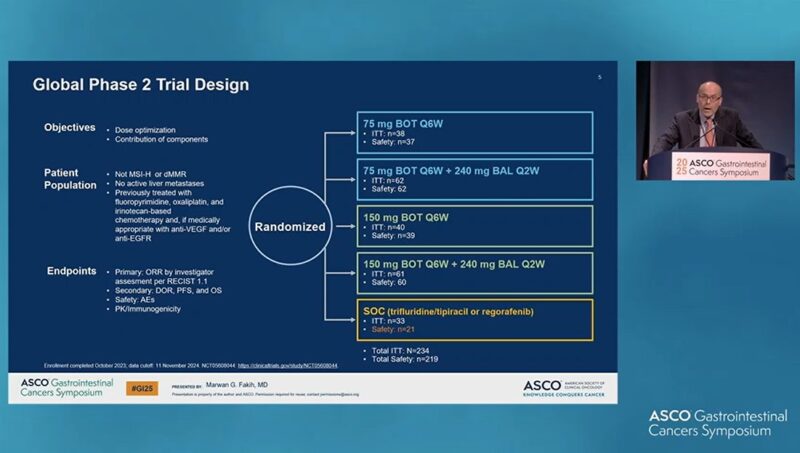
“Marwan Fakih, from City of Hope shows the very PROMISING preliminary results from a randomized, open-label, phase 2 study of BOT with or without BAL in refractory microsatellite stable metastatic colorectal cancer with no liver metastases.”
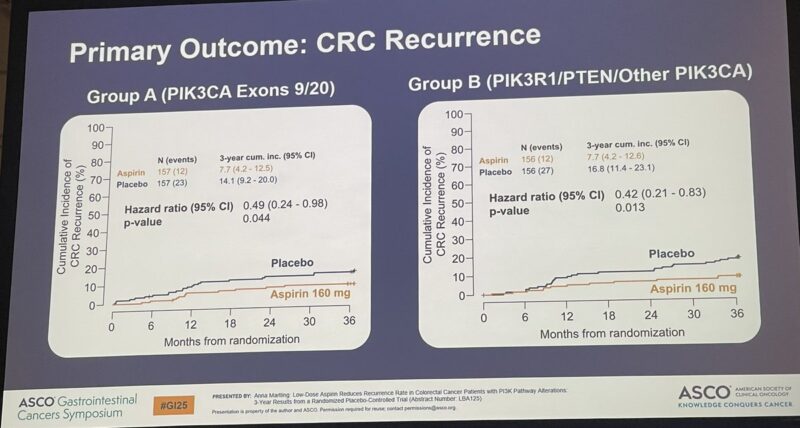
“Aspirin and Celecoxib (separate studies) shown to reduce recurrence in CRC.
Aspirin effect seems to be specific to PI3K mutated tumors.
50% reduction with aspirin alone!!!
My clinic next week.”
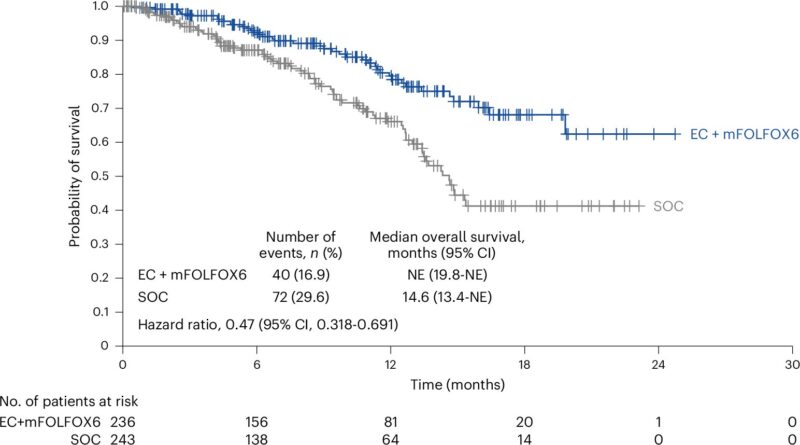
“Woah! We have a new SOC for 1L MSS CRC with BRAF V600E mt -BREAKWATER data presented by Dr Kopetz.
EC+mFOLFOX vs SOC.
Significant improvement in ORR: 60% vs 40%.
12-month OS 79% vs 66%, median NR.
These are great results! Recent FDA accelerated approval is right on the money.
New SOC!!! (without a doubt).”

“Exciting data from BESPOKE CRC indicates that ctDNA based surveillance can lead to an increased role for metastasis-directed therapy in colorectal cancer.”

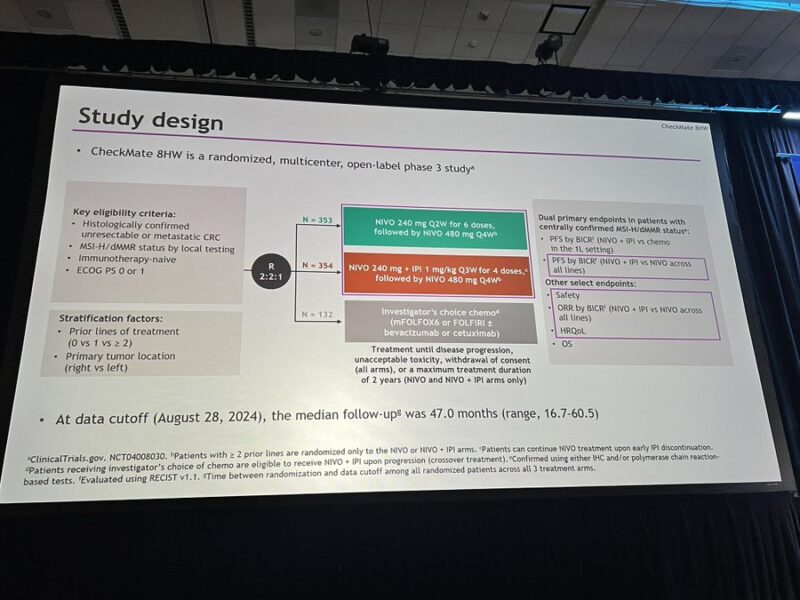
“Much awaited nivo/ipi vs nivo data from CheckMate 8HW in 1L MSI-H mCRC – nivo/ipi better in PFS and ORR across all lines of therapy with expected increase in toxicity GI25
Concomitant publication in the Lancet today for more details.”
“Sharing during GI25 with extraordinary clinical scientists including Pamela Kunz and Crystal Delinger CEO, National Comprehensive Cancer Network (NCCN).
Our PanOncology investigators including Noridza Rivera presented 5 scientific abstracts.
- long term follow up with KRAS+, colon cancer.
- ctDNA analysis for surveillance.
- MET-inhibitors for Stage IV colon cancer.
- anti-PD1 therapies for 1st line therapy for gastric cancer.”

“As GI-ASCO comes to a close I think there are a few takeaways.
1. Progress continues to march forward. dMMR disease is increasingly a systemically managed disease with excellent cure fraction (embrace CTLA4).
2. IO is breaking into MSS CRC.
3. The undruggable is becoming druggable.
4. Aspirin and Celecoxib may have clinical benefit!?
5. ctDNA is here to stay, but we need to identify actionable strategies.”
“Congrats Daniel Haldar for your ASCO GI25 master class on where we are and where we are heading for cancer vaccines in MSS CRC – excited to see where you lead the field to end cancer for our crcsm patients in the years to come!”
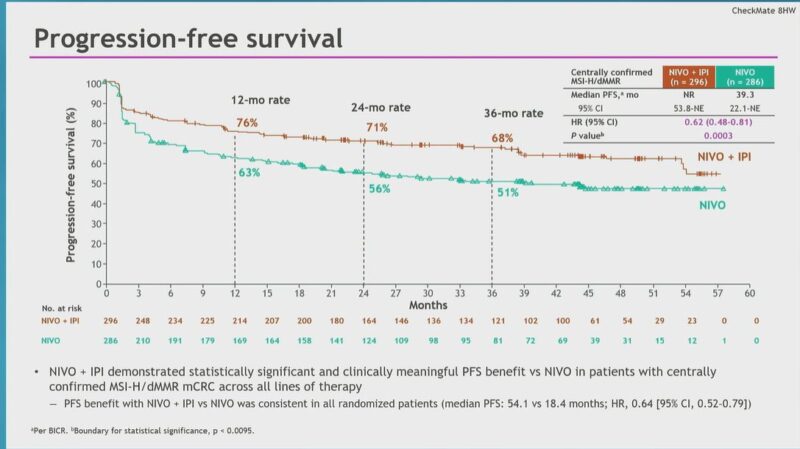
“The long awaited nivo/ipi vs nivo results.
Checkmate8HW in dMMR mCRC.
- ORR 71% vs 58%
- 3 year PFS 68% vs 51 %, HR 0.62
- no new safety signals
New SOC for dMMR mCRC.
Where does single agent pembro fit in? Would probably use if unable to get doublet.”
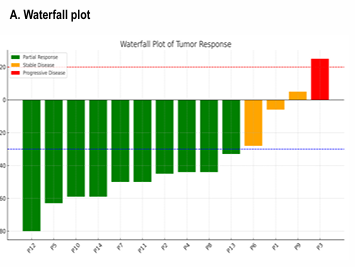
“GI/ASCO: We presented our Phase 1 BOT/BAL with FOLFOX in MSS MCRC. 14 patients on the phase 1 study. 10 had prior Ox and 12 had prior chemo, yet ORR was 10/14 patients (majority of the patients had liver mets). The combination was safe with 2 doses of BOT + Q2w FOLFOXBEV/BAT.”
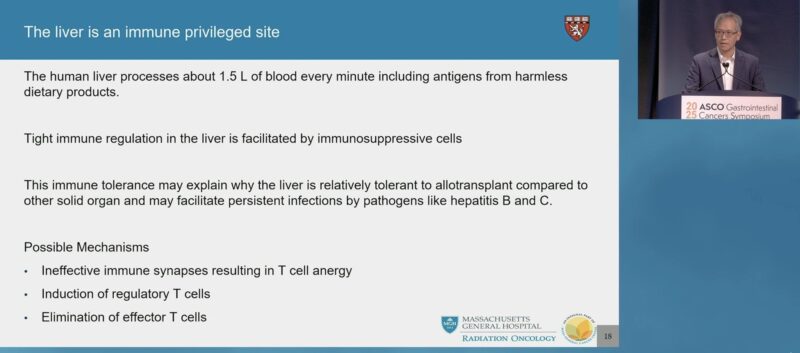
“Save the best for last! My mentor Ted Hong discusses multimodal treatment for optimizing immune response in mCRC.
Liver metastasis appear to decrease IO efficacy.
Look forward to upcoming Ph III RCT assessing addition of RT to stimulate immune response in MSS mCRC led by Aparna Raj Parikh.”
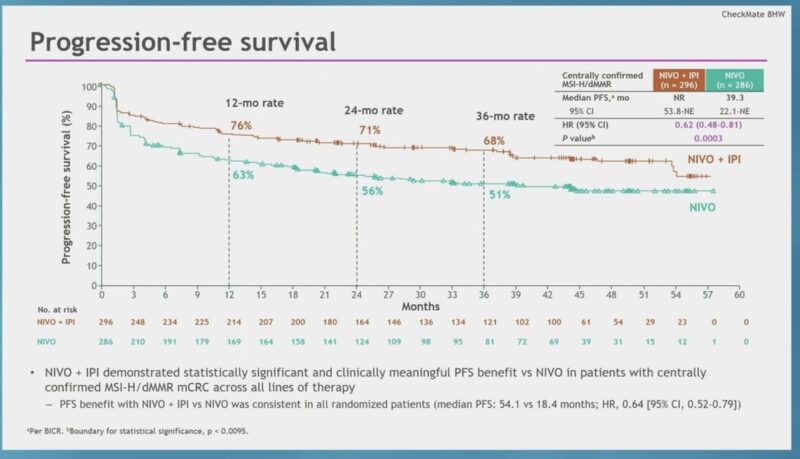
“Checkmate 8HW: dMMR/MSI-H CRC 1L.
3Y PFS: Nivo/Ipi 68% vs Nivo 51% (HR 0.62).
Groundbreaking—best 3Y PFS in any phase 3 advanced solid tumor IO study!
•Biomarker harmonization in daily practice is key given ITT vs centrally confirmed cohort differences.
•Combo shows superior efficacy, but mono offers 3Y progression-free for half, with less tox.
•Urgent need for new biomarkers as traditional factors in subgroup analysis fail to guide.
Game-changer, but still work to do!”
ASCO:
“Thank you for making GI25 a success! This year’s event highlighted groundbreaking research, transformative collaborations, and the strength of our GI cancer care community. We look forward to seeing you January 8–10, 2026, in San Francisco!”


