The treatment of metastatic hormone-sensitive prostate cancer (mHSPC) has evolved significantly with the use of androgen deprivation therapy (ADT) combined with androgen receptor pathway inhibitors (ARPI) and chemotherapy. While these therapies have improved overall survival, they also cause metabolic side effects, such as insulin resistance and dyslipidemia, which impact patients’ quality of life.
Metformin, a widely used diabetes medication, has shown potential to address these metabolic issues and possibly offer anti-cancer effects. The STAMPEDE trial aimed to assess the addition of metformin to standard treatment in mHSPC patients. This article explores the trial’s findings on metformin’s impact on survival and metabolic health.
Title: Metformin for patients with metastatic prostate cancer starting androgen deprivation therapy: a randomised phase 3 trial of the STAMPEDE platform protocol
Published in Lancet Oncology , July 2025
Background
Metastatic hormone-sensitive prostate cancer (mHSPC) is a major health concern, with androgen deprivation therapy (ADT) remaining the cornerstone of treatment. Over the past decade, combining ADT with androgen receptor pathway inhibitors (ARPI) and docetaxel has become the standard of care, improving overall survival (OS) for these patients, with median survival now exceeding 60 months. However, long-term ADT leads to adverse metabolic effects such as insulin resistance, dyslipidemia, and body composition changes, which increase cardiovascular risk.
Given these challenges, adjunct therapies to mitigate these effects are critical. Metformin, a drug primarily used to treat type 2 diabetes, has garnered attention due to its potential to improve metabolic health and its anti-cancer properties, particularly its effects on insulin sensitivity and androgen receptor expression. This article focuses on the trial, which evaluated the addition of metformin to standard treatment in patients with high-risk or metastatic prostate cancer.
Methods and Study Design
The STAMPEDE trial is a multi-arm, multi-stage platform study designed to evaluate multiple treatments for high-risk and metastatic prostate cancer. The specific study by Silke Gillessen and colleagues investigated the role of metformin as an adjunct to standard-of-care treatments in non-diabetic patients with mHSPC. A total of 1874 patients were randomly assigned in a 1:1 ratio to receive either the standard of care (ADT with or without radiotherapy, docetaxel, or ARPI) or standard of care plus metformin at a dose of 850 mg twice daily. The primary endpoint was overall survival, and secondary endpoints included metabolic parameters, progression-free survival (PFS), failure-free survival (FFS), prostate cancer-specific survival (PCSS), and toxicity.
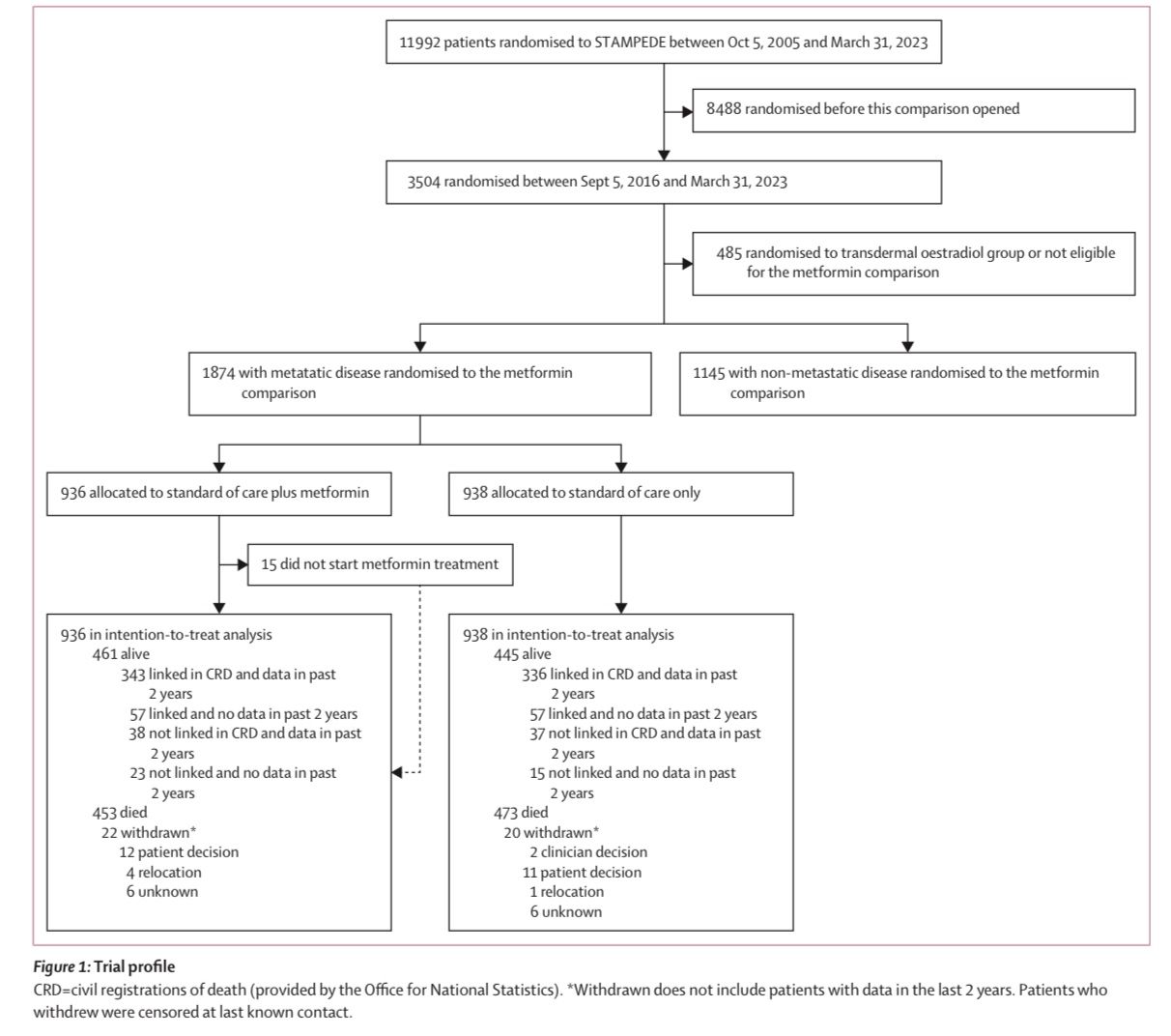
The study population consisted of patients with newly diagnosed, high-risk, or metastatic hormone-sensitive prostate cancer, including those receiving ADT alone or in combination with docetaxel and/or ARPI. Of the 1874 patients, 15% were treated with ADT alone, 82% received ADT plus docetaxel, and 3% received ADT plus ARPI.
Results
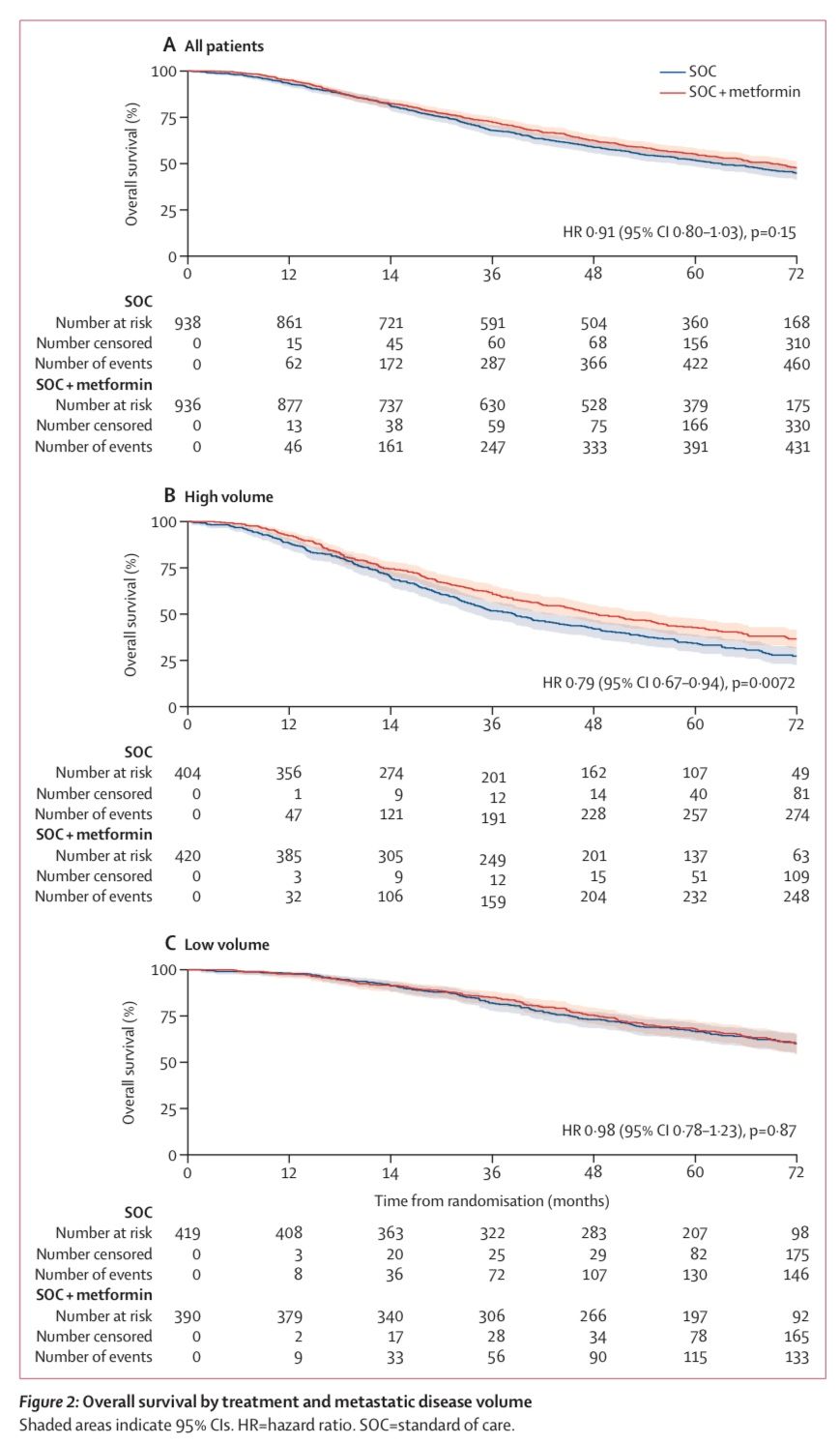
At a median follow-up of 60 months, the trial did not demonstrate a significant improvement in overall survival (OS) with the addition of metformin to standard care.
Median Overall Survival (OS):
- Metformin group: 67.4 months (IQR: 32.5 months to not reached)
- Standard of care group: 61.8 months (IQR: 29.7 months to not reached)
- Hazard Ratio (HR): 0.91, 95% CI: 0.80–1.03, P = 0.15 (no statistically significant difference)
Secondary endpoints, including prostate cancer-specific survival (PCSS), progression-free survival (PFS), metastatic progression-free survival (MPFS), and failure-free survival (FFS), also showed no significant differences between the metformin and standard care groups.
However, metformin was associated with significant metabolic benefits. Patients receiving metformin had:
- Less weight gain compared to the standard care group
- Lower fasting glucose concentrations
- Lower total cholesterol, LDL cholesterol, and glycated hemoglobin (HbA1c)
- Reduced waist circumference
At 104 weeks, these metabolic improvements were consistently observed, suggesting that metformin may help mitigate the metabolic side effects of ADT.
Key Findings
- The addition of metformin to standard care did not improve overall survival (OS), prostate cancer-specific survival (PCSS), or progression-free survival (PFS).
- Metformin treatment was associated with significant improvements in metabolic parameters, including weight gain, fasting glucose levels, cholesterol levels, and waist circumference.
- There were no significant differences in failure-free survival (FFS) or metastatic progression-free survival (MPFS) between the two groups.
Key Takeaway Messages
- Metformin did not improve overall survival or prostate cancer-related outcomes in patients with mHSPC, which was the primary objective of the trial.
- Despite the lack of survival benefit, metformin provided substantial metabolic benefits, including reduced weight gain, improved insulin sensitivity, and better lipid profiles, which are critical in managing the long-term effects of ADT.
- Given the increasing life expectancy of patients with mHSPC, mitigating the metabolic effects of ADT is important for improving patients’ quality of life and reducing cardiovascular risk.
- The findings highlight the potential of metformin as an adjunctive treatment to manage metabolic health in prostate cancer patients, though further investigation is needed to determine its role in improving cardiovascular outcomes.
Conclusion
The trial, while not showing survival benefits, demonstrated that metformin may help manage the metabolic consequences of androgen deprivation therapy in patients with metastatic hormone-sensitive prostate cancer. These metabolic benefits, including weight management and improved glucose and lipid profiles, are important for patient health, particularly given the cardiovascular risks associated with ADT. Although metformin did not improve survival outcomes, it remains a reasonable adjunctive therapy for improving metabolic health in these patients. Future studies are needed to evaluate whether these metabolic improvements translate into long-term reductions in cardiovascular morbidity and whether metformin should be recommended for all patients undergoing ADT or only those with pre-existing metabolic dysfunction.
You can read the full abstract here
Written by Sona Karamyan, MD