At the MASCC 2025 Annual Meeting, Sarah Bayrakdarian presented the long-term results of a randomized controlled trial evaluating Mepitel Film (MF) versus standard of care (SOC) in patients receiving radiation therapy (RT) for breast cancer. The study is a secondary analysis of a multicenter Phase III trial originally conducted across three Ontario centers. The primary trial previously demonstrated that MF significantly reduced the incidence of grade 2–3 acute radiation dermatitis (ARD) by 45% compared to SOC. The current analysis aimed to assess whether these benefits extended to chronic radiation dermatitis (CRD) and other long-term skin-related patient-reported outcomes.
Background
Breast cancer remains the most commonly diagnosed cancer among women worldwide. Adjuvant radiation therapy is a cornerstone in breast cancer treatment, known to reduce recurrence risk. However, it frequently leads to ARD within weeks of treatment, manifesting as erythema, swelling, itching, pain, or even moist desquamation. CRD, in contrast, may develop months or years after RT, presenting with more permanent changes such as fibrosis, pigmentation changes, atrophy, and telangiectasia. These late effects can substantially impair quality of life. Given the promising short-term findings with MF, there is clinical interest in determining its long-term safety and effectiveness in reducing CRD.
Study Design and Methods of Mepitel Film
This secondary analysis focused on long-term patient-reported outcomes collected from patients who had participated in the original RCT comparing MF to an aqueous moisturizer (SOC). Participants were contacted via telephone at 6, 12, and 24 months post-radiation to complete two validated tools: the Skin Symptom Assessment (SSA) and the Radiation-Induced Skin Reaction Assessment Scale (RISRAS). Statistical analysis compared post-treatment scores with baseline using generalized estimation equations (Poisson distribution and log link function). Bonferroni correction was applied, with a significance threshold of p<0.001.
Results
When comparing MF to SOC over time, no significant differences were observed in long-term SSA or RISRAS scores. Graphical analyses showed comparable mean burning sensation scores between arms, even when stratified by baseline ARD severity (CTCAE grade 0–1 vs. 2–3).
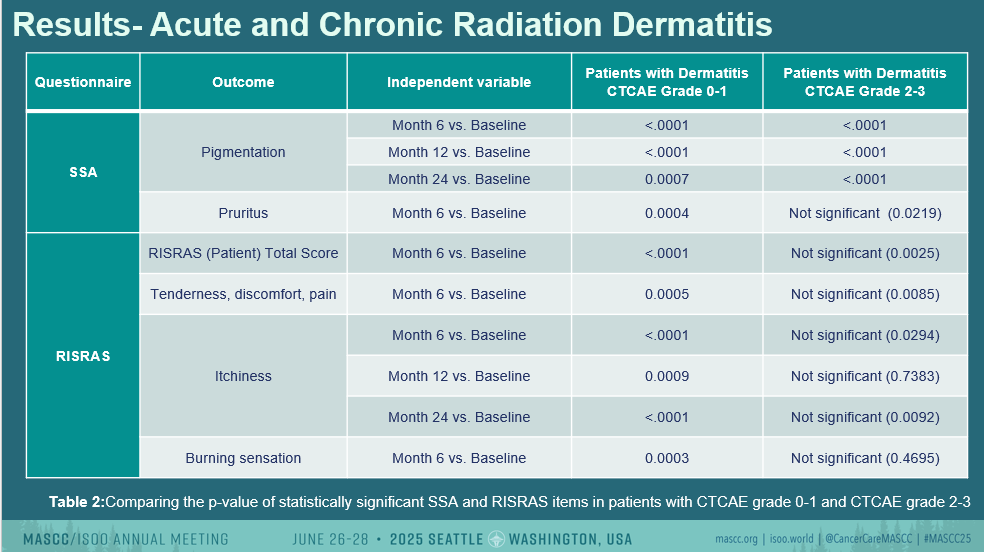
However, independent of treatment arm, several chronic skin changes were noted:
- Pigmentation scores increased significantly at 6, 12, and 24 months post-RT (all p<0.0001).
- Pruritus was significantly elevated at 6 months (p<0.0001).
- Tenderness/discomfort/pain and burning sensation were higher at 6 months per patient-reported RISRAS (p<0.0001 and p=0.0003, respectively).
- Itchiness also increased at all timepoints, with significant differences from baseline (p<0.0001 to p=0.0007).
- The total patient RISRAS score was significantly elevated at 6 and 24 months.
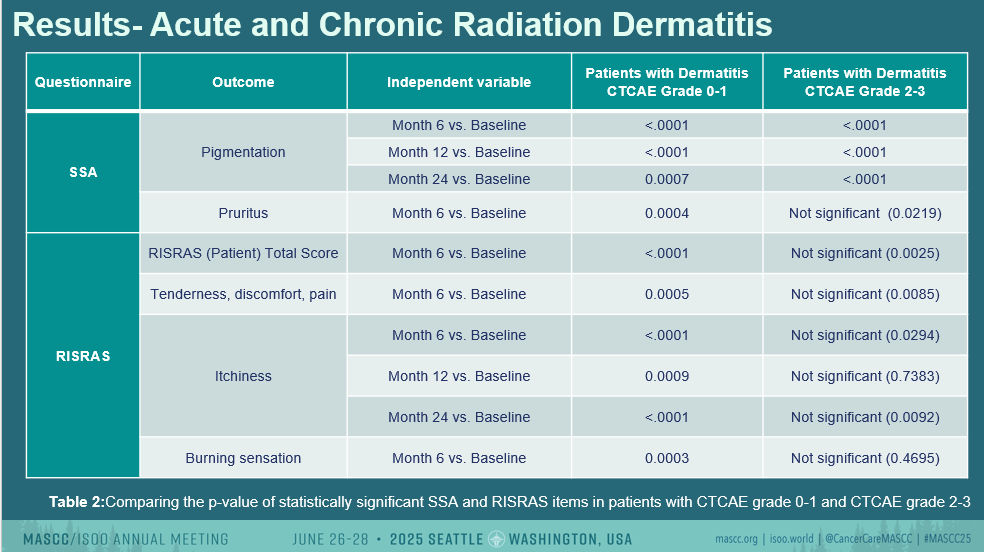
Importantly, these persistent symptoms were also present in patients who did not develop clinically documented ARD during treatment, suggesting that chronic symptoms can arise even in the absence of visible acute skin damage.
Discussion
The analysis revealed no statistically significant differences in long-term CRD severity between the MF and SOC arms. This trend held even when stratifying by initial ARD status. These findings suggest that while MF is effective in reducing acute skin toxicity, its benefits may not extend into the chronic phase, at least from the patient-reported perspective. The observed disconnect between ARD and CRD reinforces the complexity of long-term radiation effects, which may be influenced by a broader array of biological or individual factors.
Limitations included:
- Incomplete clinician assessments during long-term follow-up due to COVID-19.
- Lack of patient blinding to treatment assignment.
- Reduced response rates during follow-up telephone interviews compared to baseline.
Conclusion
There were no significant differences in long-term patient-reported skin outcomes between the MF and SOC groups. Nonetheless, MF remains a safe and effective option for reducing ARD in breast cancer patients undergoing RT, with no indication of worse long-term effects. Further research is warranted to understand the mechanisms and predictors of CRD in this population.


