KEYNOTE-991 is a pivotal phase III trial that explored whether adding pembrolizumab to enzalutamide and androgen deprivation therapy (ADT) could improve clinical outcomes in men with metastatic hormone-sensitive prostate cancer (mHSPC) who had not previously received next-generation hormonal agents.
With ADT and enzalutamide already established as standard of care, this study sought to test the hypothesis that immunotherapy could further enhance treatment efficacy by targeting immune resistance mechanisms. Given previous signals of activity in mCRPC and earlier-stage disease, this trial was designed to determine if early introduction of pembrolizumab could delay disease progression and improve survival in the hormone-sensitive setting.
Tilte: Pembrolizumab plus enzalutamide and androgen deprivation therapy versus placebo plus enzalutamide and androgen deprivation therapy for metastatic hormone-sensitive prostate cancer: the randomized, double-blind, phase III KEYNOTE-991 study
Authors: C. Gratzke, M. Özgüroğlu, A. Peer, M.A.N. Sendur, M. Retz, J.C. Goh, W. Loidl, G. Jayram, S.-S. Byun, C. Kwak, M. Kwiatkowski, R.M. Kopp, J.C.V. Limón, J.F.E. Penagos, U. De Giorgi, K.M. da Trindade, C. Niu, Y. Liu, C.H. Poehlein, J.M. Piulats.
Published in Annals of Oncology, May 2025
Background
Prostate cancer remains one of the leading causes of cancer-related mortality in men, and optimizing treatment strategies for patients with metastatic hormone-sensitive prostate cancer (mHSPC) is a continuing challenge in clinical oncology. While androgen deprivation therapy (ADT) combined with next-generation hormonal agents such as enzalutamide has become standard of care, long-term control remains suboptimal for many patients.
Immune checkpoint inhibitors like pembrolizumab have shown limited efficacy as monotherapy in prostate cancer, but preclinical data suggest potential synergy when combined with androgen receptor pathway inhibition. The phase III KEYNOTE-991 trial was designed to test whether adding pembrolizumab to enzalutamide and ADT could improve survival and disease control in men with mHSPC who had not previously received next-generation hormonal therapy.
Methods
This was a multicenter, open-label, phase III clinical trial evaluating the combination of pembrolizumab, enzalutamide, and ADT in patients with mHSPC who had not previously received next-generation hormonal agents. The primary endpoint was progression-free survival (PFS), with secondary endpoints including overall survival (OS), objective response rate (ORR), and safety profiles.
Study Design
KEYNOTE-991 enrolled 1,251 patients with histologically confirmed prostate adenocarcinoma and at least two bone and/or visceral metastases. Patients were randomized 1:1 to receive pembrolizumab (200 mg every 3 weeks, up to 35 cycles) or placebo, both in combination with daily enzalutamide (160 mg) and continuous ADT. Stratification factors included prior docetaxel use and disease volume.
The dual primary endpoints were radiographic progression-free survival (rPFS) and overall survival (OS). Secondary endpoints included time to first subsequent anticancer therapy (TFST), time to first symptomatic skeletal-related event (TTSSRE), objective response rate (ORR), PSA response, time to PSA progression, and patient-reported outcomes (PROs).
Results
The phase III KEYNOTE-991 trial failed to meet its primary endpoint of improved rPFS and showed no survival advantage for pembrolizumab plus enzalutamide and ADT in men with NHA-naive mHSPC. The increased toxicity profile further diminishes the clinical appeal of this combination.
These findings align with other phase III trials such as KEYNOTE-641 and IMbassador250, which also failed to show benefit of immune checkpoint inhibitors in broader prostate cancer populations. Future research should focus on identifying molecularly selected subgroups who may benefit from immunotherapy in prostate cancer.
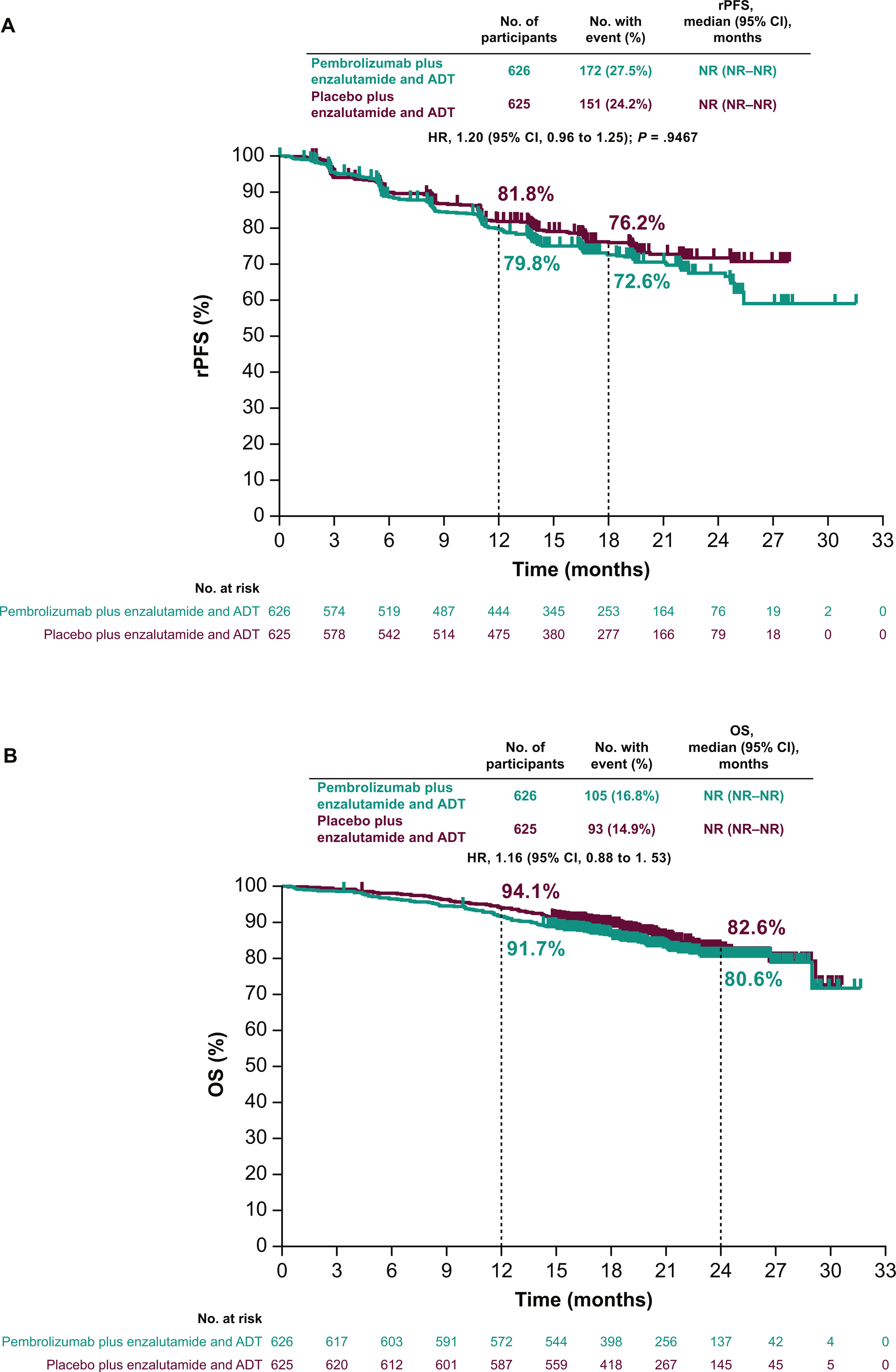
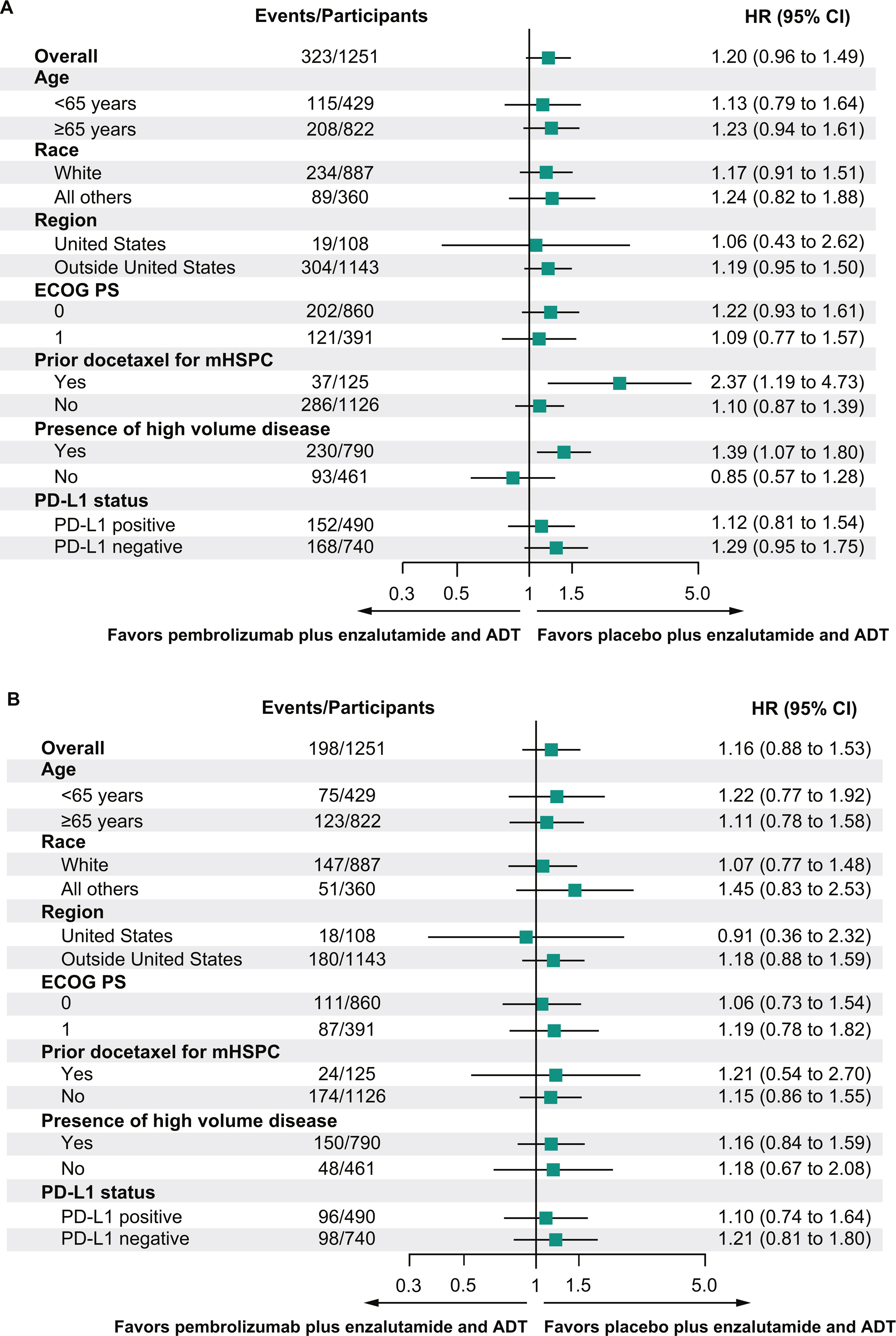
- Median follow-up was 21.1 months at the first prespecified interim analysis.
- Median rPFS was not reached in either arm (HR, 1.20; 95% CI, 0.96–1.49; P = 0.9467).
- No favorable trend in OS was observed (HR, 1.16; 95% CI, 0.88–1.53).
- Objective response rate (ORR): 65.7% in pembrolizumab arm vs 71.8% with placebo.
- PSA response rate (≥50% reduction): 90.3% with pembrolizumab vs 93.0% with placebo.
- Time to PSA progression: HR, 0.92; median not reached in both arms.
- TFST: HR, 1.24 (no benefit).
- TTSSRE: HR, 0.89 (no benefit).
Key Findings
The addition of pembrolizumab to enzalutamide and ADT in mHSPC did not demonstrate clinical benefit in terms of progression-free or overall survival. Although the hypothesis of enhancing immunotherapy efficacy via AR pathway inhibition was biologically plausible, the trial results did not support its use in unselected mHSPC populations.
Furthermore, the combination led to a marked increase in toxicity, including immune-mediated side effects and higher treatment discontinuation rates.
Key Takeaway Messages
- Pembrolizumab did not improve rPFS or OS when added to enzalutamide and ADT.
- ORR, PSA response, and time to PSA progression were similar between groups.
- Safety concerns were notable: Grade ≥3 adverse events occurred in 61.9% of pembrolizumab-treated patients versus 38.1% in the placebo group.
- Treatment discontinuation due to adverse events was significantly higher in the pembrolizumab arm (33.4% vs 8.2%).
- Immune-related adverse events (43.2% vs 7.5%) and severe rash (25.1% vs 9.3%) were more frequent with pembrolizumab.
- Median time to pain progression and quality-of-life outcomes were similar between arms.
You cn read the full article here.